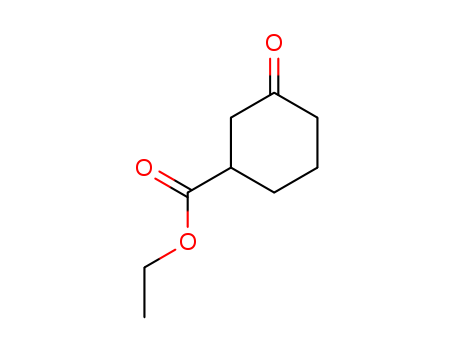
33668-25-6
- Product Name:Ethyl 3-Oxocyclohexane-1- Carboxylate
- Molecular Formula:C9H14O3
- Purity:99%
- Molecular Weight:170.208
Product Details;
CasNo: 33668-25-6
Molecular Formula: C9H14O3
Factory Supply Ethyl 3-Oxocyclohexane-1- Carboxylate 33668-25-6 Export with Efficient Shipping
- Molecular Formula:C9H14O3
- Molecular Weight:170.208
- Vapor Pressure:0.0238mmHg at 25°C
- Refractive Index:1.464
- Boiling Point:248.8 °C at 760 mmHg
- Flash Point:103.8 °C
- PSA:43.37000
- Density:1.084 g/cm3
- LogP:1.30880
ETHYL 3-OXOCYCLOHEXANE-1-CARBOXYLATE(Cas 33668-25-6) Usage
|
Uses |
Ethyl Cyclohexanone-β-carboxylate is an intermediate used to prepare carbazole-carboxamides with selective JAK2 inhibitory activities. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 110, p. 2565, 1988 DOI: 10.1021/ja00216a033 |
InChI:InChI=1/C9H14O3/c1-2-12-9(11)7-4-3-5-8(10)6-7/h7H,2-6H2,1H3
33668-25-6 Relevant articles
A photo-reagent system of benzimidazoline and Ru(bpy)3Cl 2 to promote hexenyl radical cyclization and Dowd-Beckwith ring-expansion of α-halomethyl-substituted benzocyclic 1-alkanones
Hasegawa, Eietsu,Tateyama, Minami,Hoshi, Tsuneaki,Ohta, Taku,Tayama, Eiji,Iwamoto, Hajime,Takizawa, Shin-Ya,Murata, Shigeru
, p. 2776 - 2783 (2014)
A combination of 2-aryl substituted 1,3-...
A model of the methylmalonyl isomerase reaction.
Lowe,Ingraham
, p. 3801 - 3802 (1971)
-
Rapid Enantioselective and Diastereoconvergent Hybrid Organic/Biocatalytic Entry into the Oseltamivir Core
Tiwari, Virendra K.,Powell, Douglas R.,Broussy, Sylvain,Berkowitz, David B.
, p. 6494 - 6503 (2021/05/06)
A formal synthesis of the antiviral drug...
Phosphinous Acid Platinum Complex as Robust Catalyst for Oxidation: Comparison with Palladium and Mechanistic Investigations
Membrat, Romain,Vasseur, Alexandre,Martinez, Alexandre,Giordano, Laurent,Nuel, Didier
supporting information, p. 5427 - 5434 (2018/10/20)
Secondary phosphine oxides proved to be ...
Synthesis of gem-difluorocyclopentane/hexane building blocks
Melnykov, Kostiantyn P.,Nosik, Pavel S.,Kurpil, Bohdan B.,Sibgatulin, Dmitriy A.,Volochnyuk, Dmitriy M.,Ryabukhin, Sergey V.,Grygorenko, Oleksandr O.
, p. 60 - 66 (2017/05/17)
An approach to the preparation of gem-di...
Biocatalytic synthesis of chiral cyclic γ-oxoesters by sequential C-H hydroxylation, alcohol oxidation and alkene reduction
Brenna, Elisabetta,Crotti, Michele,Gatti, Francesco G.,Monti, Daniela,Parmeggiani, Fabio,Pugliese, Andrea,Tentori, Francesca
, p. 5122 - 5130 (2017/11/09)
A three-step biocatalytic procedure is d...
33668-25-6 Process route
-
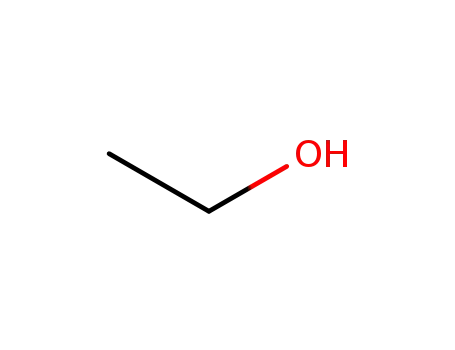
-
64-17-5
ethanol

-
-
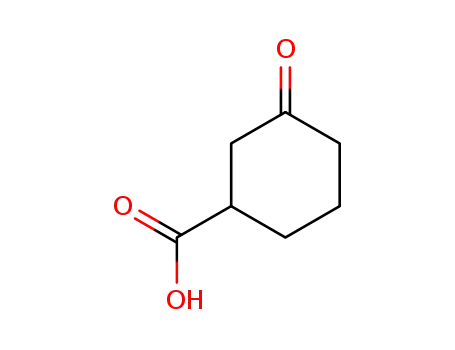
16205-98-4
3-oxo-cyclohexanecarboxylic acid

-
-
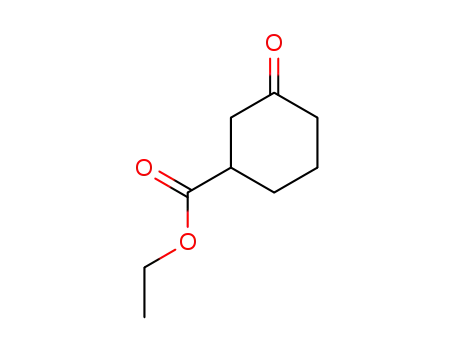
33668-25-6
ethyl 3-oxocyclohexanecarboxylate
| Conditions | Yield |
|---|---|
|
With
toluene-4-sulfonic acid;
In
toluene;
Inert atmosphere;
Reflux;
|
100% |
|
With
thionyl chloride;
at 15 - 20 ℃;
|
75% |
|
With
hydrogenchloride;
|
|
|
With
toluene-4-sulfonic acid;
In
toluene;
Reflux;
|
7.5 g |
|
With
toluene-4-sulfonic acid;
In
toluene;
Reflux;
Dean-Stark;
|
7.5 g |
-
-
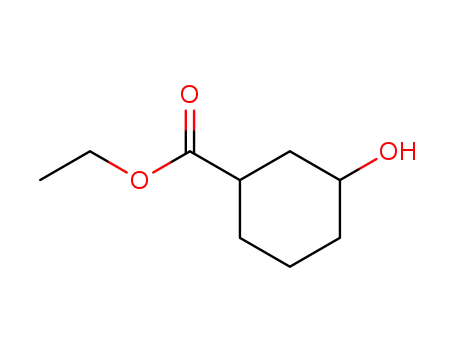
94160-25-5
hydroxy-3 cyclohexane carboxylate d'ethyle

-

-
33668-25-6
ethyl 3-oxocyclohexanecarboxylate
| Conditions | Yield |
|---|---|
|
With
chromium(VI) oxide; sulfuric acid;
In
acetone;
|
95% |
|
With
Dess-Martin periodane;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
95% |
|
With
chromium(VI) oxide; sulfuric acid;
In
water; acetone;
at 5 - 10 ℃;
|
93.5% |
|
With
di-μ-chlorotetrakys-[(RP)-tert-butylphenylphosphinito-κ-P]diplatinate(2-); methyl vinyl ketone; sodium hydroxide;
In
water; toluene;
for 16h;
Schlenk technique;
Inert atmosphere;
Heating;
|
72% |
33668-25-6 Upstream products
-
64-17-5

ethanol
-
16205-98-4

3-oxo-cyclohexanecarboxylic acid
-
99-06-9
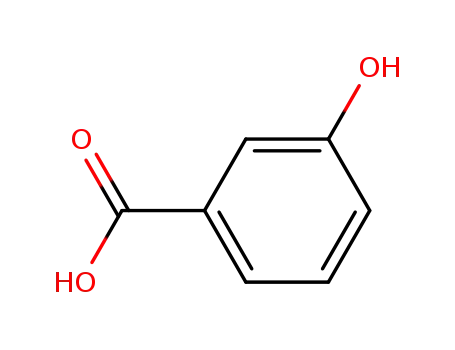
3-Carboxyphenol
-
21531-47-5
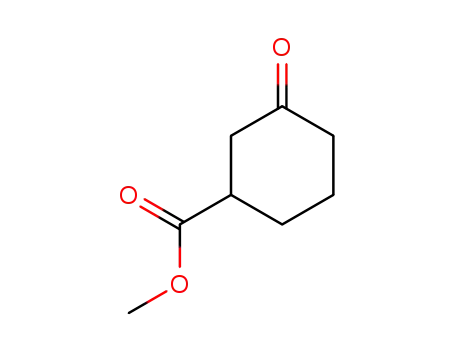
3-oxo-cyclohexanecarboxylic acid methyl ester
33668-25-6 Downstream products
-
93428-11-6
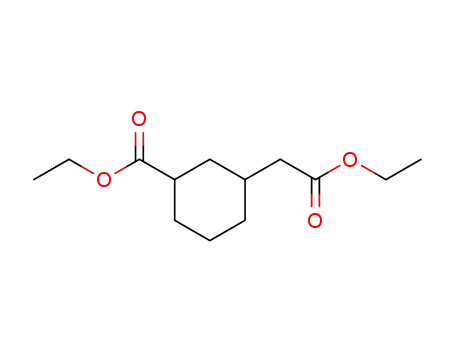
(3-ethoxycarbonyl-cyclohexyl)-acetic acid ethyl ester
-
163980-32-3
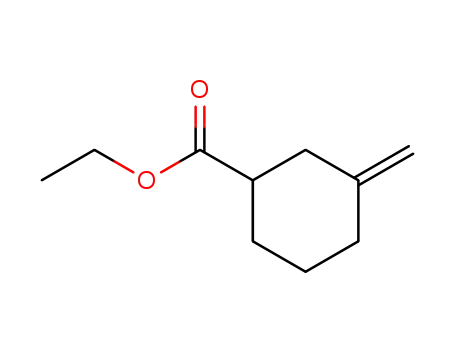
Ethyl 3-methylenecyclohexanecarboxylate
-
6553-08-8
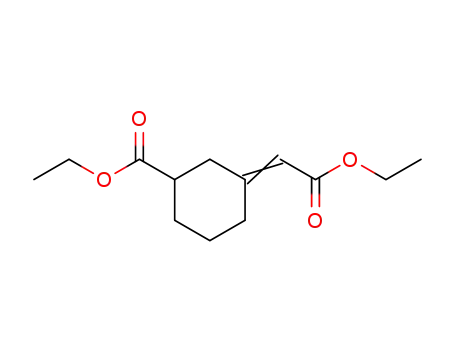
3-Ethoxycarbonyl-cyclohexyliden-essigsaeure-ethylester
-
6553-12-4
bicyclo[3.2.1]octan-6-one
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
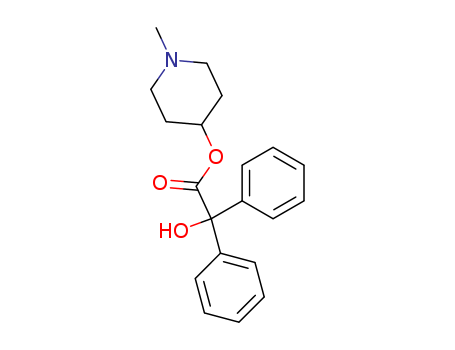
1-methylpiperidin-4-yl hydroxy(diphenyl)acetate
CAS:3608-67-1
-
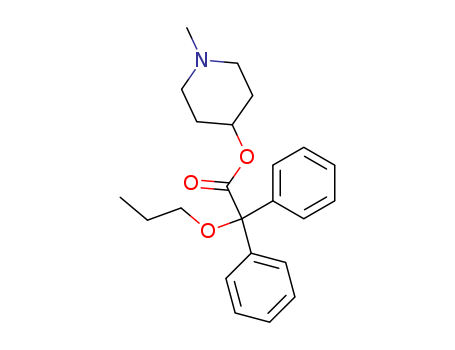
Propiverine
CAS:60569-19-9