71-58-9
- Product Name:Medroxyprogesterone Acetate
- Molecular Formula:C24H34O4
- Purity:99%
- Molecular Weight:386.532
Product Details;
CasNo: 71-58-9
Molecular Formula: C24H34O4
Appearance: white crystalline powder
Buy High Quality High Purity 99% Medroxyprogesterone Acetate 71-58-9 Best Price
- Molecular Formula:C24H34O4
- Molecular Weight:386.532
- Appearance/Colour:white crystalline powder
- Vapor Pressure:3.85E-10mmHg at 25°C
- Melting Point:206-207 °C(lit.)
- Refractive Index:48 ° (C=1, Dioxane)
- Boiling Point:496.4 °C at 760 mmHg
- Flash Point:213.2 °C
- PSA:60.44000
- Density:1.13 g/cm3
- LogP:4.51270
Medroxyprogesterone 17-acetate(Cas 71-58-9) Usage
|
Therapeutic Function |
Progestin |
|
World Health Organization (WHO) |
A depot preparation containing 150 mg medroxyprogesterone acetate was introduced over 20 years ago for use as a long-acting injectable contraceptive. Subsequently, positive results of carcinogenicity studies carried out in beagle bitches led to refusal of registration in the United States. These findings were later considered irrelevant to contraceptive use in women and the drug was approved by the Food and Drug Administration. Menstrual irregularities are the most common adverse effect associated with depot medroxyprogesterone acetate. Risk-benefit judgements differ significantly from country to country, having regard to differing national circumstances. The preparation is, however, widely available and is included in the WHO Model List of Essential Drugs. (Reference: (WHTAC4) The Use of Essential Drugs, 4th Report of the WHO Expert Committee, 796, , 1990) |
|
Air & Water Reactions |
Medroxyprogesterone 17-acetate is sensitive to prolonged exposure to air and light. Insoluble in water. |
|
Hazard |
Possible carcinogen. |
|
Fire Hazard |
Flash point data for Medroxyprogesterone 17-acetate are not available; however, Medroxyprogesterone 17-acetate is probably combustible. |
|
Biochem/physiol Actions |
Medroxyprogesterone 17-acetate (MPA) is a synthetic progestin used as a contraceptive, in hormone replacement therapy and for the treatment of endometriosis. It is a more potent progestin that the nonacetylated form. |
|
Safety Profile |
Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Human systemic effects by intravenous route: increased intraocular pressure. Human teratogenic effects by an unspecified route: developmental abnormalities of the urogenital system. Human reproductive effects by multiple routes: spermatogenesis, menstrual cycle changes or dlsorders, postpartum effects, female fertility effects, abortion, newborn behavioral effects. Human mutation data reported. Experimental reproductive effects. A drug for the treatment of secondary amenorrhoea and dysfunctional uterine bleeding. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Veterinary Drugs and Treatments |
In cats, MPA has been used when either castration is ineffective or undesirable to treat sexually dimorphic behavior problems such as roaming, inter-male aggressive behaviors, spraying, mounting, etc. MPA has also been used as a tranquilizing agent to treat syndromes such as feline psychogenic dermatitis and alopecia, but treatment with “true” tranquilizing agents may be preferable. In humans, parenteral MPA has been used as a long-acting contraceptive in females, to decrease sexually deviant behavior in males, and as an antineoplastic agent for some carcinomas (see Pharmacology section above). Oral MPA is used in human females to treat secondary amenorrhea and to treat abnormal uterine bleeding secondary to hormone imbalances. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: metabolism of progestogens accelerated by griseofulvin and rifamycins (reduced contraceptive effect). Anticoagulants: progestogens antagonise anticoagulant effect of phenindione and may enhance or reduce effect of coumarins. Antidepressants: contraceptive effect reduced by St John’s Wort - avoid. Antiepileptics: metabolism accelerated by carbamazepine, eslicarbazepine, fosphenytoin, lamotrigine, oxcarbazepine, perampanel, phenytoin, phenobarbital, primidone, rufinamide and topiramate (reduced contraceptive effect); concentration of lamotrigine reduced. Antivirals: contraceptive effect possibly reduced by efavirenz; metabolism accelerated by nevirapine (reduced contraceptive effect). Aprepitant: possible contraceptive failure. Bosentan: possible contraceptive failure. Ciclosporin: progestogens inhibit metabolism of ciclosporin (increased plasma concentration). Cytotoxics: possibly reduced contraceptive effect with crizotinib dabrafenib, olaparib and vemurafenib. Dopaminergics: concentration of selegiline increased - avoid. Fosaprepitant: possible contraceptive failure. Lumacaftor: possible contraceptive failure. Ulipristal: contraceptive effect possibly reduced |
|
Metabolism |
Among the first of these substituted 17α-acetoxyprogesterone analogues to be utilized therapeutically was medroxyprogesterone acetate, a 6α-methyl progesterone analogue. This analogue is 25-fold more active than ethisterone. Following oral administration, medroxyprogesterone acetate is completely and rapidly deacetylated by first-pass metabolism to medroxyprogesterone. Medroxyprogesterone is extensively metabolized via pathways similar to those for progesterone, except for 6α-hydroxylation. Most medroxyprogesterone acetate metabolites are excreted in the urine, primarily as glucuronide conjugates. Plasma protein binding for medroxyprogesterone is approximately 86%, primarily to serum albumin, with no binding to SHBG. |
|
|
|
|
Definition |
ChEBI: Medroxyprogesterone acetate is an acetate ester resulting from the formal condensation of the 17alpha-hydroxy group of medroxyprogesterone with the carboxy group of acetic acid. A widely used progestin in menopausal hormone therapy and in progestogen-only birth control. It has a role as a progestin, an androgen, a female contraceptive drug, a synthetic oral contraceptive, an adjuvant, an inhibitor, an antioxidant and an antineoplastic agent. It is a steroid ester, an acetate ester, a 20-oxo steroid, a 3-oxo-Delta(4) steroid and a corticosteroid. It is functionally related to a medroxyprogesterone. |
|
Brand name |
Amen (Amarin); Curretab (Solvay Pharmaceuticals); Cycrin (ESI); Provera (Pharmacia & Upjohn);Clinovie;Cliovir;Dep0-clinover;Dep0-map;Depcorlutin;Depo-prodasone;Depo-progevera;Depo-promone;Deporone;Dugen;Farlurin;Farlutale;Gesinal;Gestapuran;Gestapuron;G-farlutal;Hysron;Intex;Luteocrin orale;Luteodione;Luteos;Lutoporal;Metigestene;Nadigest;Nogest;Onco-provera;Perlutest;Petogen;Piermap;Povera;Promone-e;Pronone;Proverone;Provest;Sindomens;Sodelut "g";Supprestal;Verafen;Veramix plus v. |
|
General Description |
Medroxyprogesterone acetate is an odorless white to off-white microcrystalline powder. It?is a synthetic, acetate derivative of the sex hormone progesterone. (NTP, 1992) |
InChI:InChI=1/C24H34O4/c1-13-10-17-18(23(4)8-6-16(27)11-19(13)23)7-9-24(5)20(17)12-21(28-15(3)26)22(24)14(2)25/h11,13,17-18,20-22H,6-10,12H2,1-5H3
71-58-9 Relevant articles
Preparation method of medroxyprogesterone acetate for perimenopausal syndrome
-
, (2022/03/17)
The invention relates to a preparation m...
Preparation method of palace progesterone
-
Paragraph 0115; 0121; 0124; 0130-0131; 0137, (2021/11/03)
The invention relates to the technical f...
Preparation method of medroxyprogesterone acetate
-
Paragraph 0012; 0028; 0033; 0036-0040, (2018/03/26)
The invention provides a preparation met...
THERAPEUTIC FOR HEPATIC CANCER
-
, (2011/02/18)
A novel pharmaceutical composition for t...
71-58-9 Process route
-
- 32634-95-0
17α-Acetoxy-6-methylene-pregn-4-ene-3,20-dione

-
- 71-58-9
Medroxyprogesterone acetate
| Conditions | Yield |
|---|---|
|
With ethanol; 5%-palladium/activated carbon; In cyclohexene; at 75 - 78 ℃; for 5h; Reagent/catalyst; Temperature;
|
87.5% |
|
With sodium acetate; cyclohexene; palladium on activated charcoal; In ethanol; water; Heating / reflux;
|
-
- 986-96-9
3,17-diacetoxy-6-methyl-pregna-3,5-dien-20-one

-
- 71-58-9
Medroxyprogesterone acetate
| Conditions | Yield |
|---|---|
|
With hydrogenchloride;
|
|
|
With hydrogenchloride; In methanol; water; at 50 ℃; Solvent; Reagent/catalyst; Temperature;
|
71-58-9 Upstream products
-
2381-48-8
6-methylpregn-5-ene-3β,17α-diol-20-one 17-acetate
-
986-96-9
3,17-diacetoxy-6-methyl-pregna-3,5-dien-20-one
-
520-85-4
Medroxyprogesterone
-
108-24-7
acetic anhydride
71-58-9 Downstream products
-
595-33-5
Megestrol acetate
-
151-68-8
6α-Methyl-17α-hydroxy-1,4-pregnadien-3,20-dion
-
520-85-4
Medroxyprogesterone
-
57-16-9
17α-acetoxy-3β-hydroxy-6α-methylpregn-4-en-20-one
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
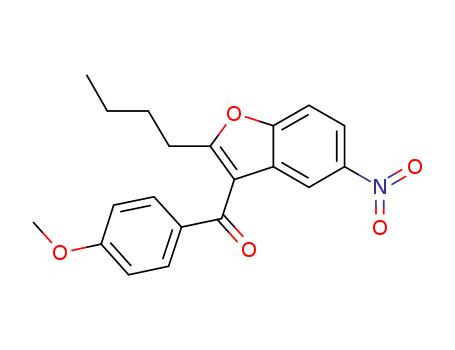
2-Butyl-3-(4-methoxybenzoyl)-5-nitrobenzofuran
CAS:141627-42-1