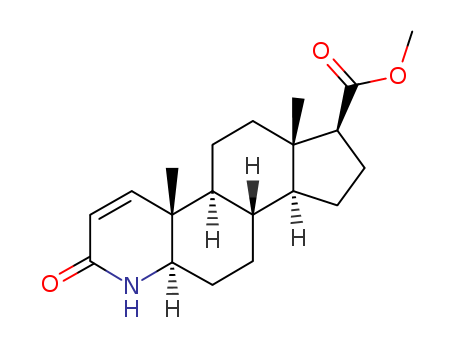
103335-41-7
- Product Name:Methyl 4-aza-5alpha-androsta-1-en-3-one-17beta-carboxylate((finasteride intermediate)
- Molecular Formula:C20H29NO3
- Purity:99%
- Molecular Weight:331.455
Product Details;
CasNo: 103335-41-7
Molecular Formula: C20H29NO3
Appearance: Off-White Solid
Hot Sale Factory Supply Methyl 4-aza-5alpha-androsta-1-en-3-one-17beta-carboxylate((finasteride intermediate) 103335-41-7 On Stock
- Molecular Formula:C20H29NO3
- Molecular Weight:331.455
- Appearance/Colour:Off-White Solid
- Vapor Pressure:0mmHg at 25°C
- Melting Point:280-282 °C (dec.)
- Refractive Index:1.525
- Boiling Point:478.615 °C at 760 mmHg
- PKA:14.15±0.70(Predicted)
- Flash Point:243.258 °C
- PSA:55.40000
- Density:1.114 g/cm3
- LogP:3.40160
Methyl 4-aza-5alpha-androsta-1-en-3-one-17beta-carboxylate (Cas 103335-41-7) Usage
|
Chemical Properties |
Off-White Solid |
| Description | Methyl 4-aza-5alpha-androsta-1-en-3-one-17beta-carboxylate((finasteride intermediate), also known as Methyl 4-aza-DHEA, is a synthetic compound that has gained significant attention in the scientific community due to its potential therapeutic and industrial applications. |
|
Uses |
Finasteride (F342000) and Dutasteride (D735000) impurity. An intermediate in the synthesis of Finasteride and Dutasteride, 5α-reductase inhibitors used for treatment of benign prostatic hyperplasia ac ne, seborrhea, female hirsutism, prostatitis, and prostatic carcinoma and other hyperandrogenetic related disorders. |
InChI:InChI=1/C20H29NO3/c1-19-10-8-14-12(13(19)5-6-15(19)18(23)24-3)4-7-16-20(14,2)11-9-17(22)21-16/h9,11-16H,4-8,10H2,1-3H3,(H,21,22)/t12-,13-,14-,15+,16+,19-,20+/m0/s1
103335-41-7 Relevant articles
A method of preparation he male amine
-
Paragraph 0010-0011, (2018/09/26)
The invention belongs to the field of ph...
Method for forming double bonds between 1-position and 2-position during synthesis of finasteride and dutasteride
-
Paragraph 0064; 0065; 0066, (2016/12/01)
The invention provides a method for form...
PROCESS FOR PREPARING ANDROSTENONE DERIVATIVES
-
Page/Page column 16, (2012/04/04)
Provided is a process for preparing andr...
METHOD OF PRODUCING 17BETA-(SUBSTITUTED)-3-OXO-DELTA 1,2-4-AZASTEROIDS AND INTERMEDIATES
-
Page 11, (2008/06/13)
The 17β-(substituted)-3-oxo-Δ1,2-azaster...
103335-41-7 Process route
-
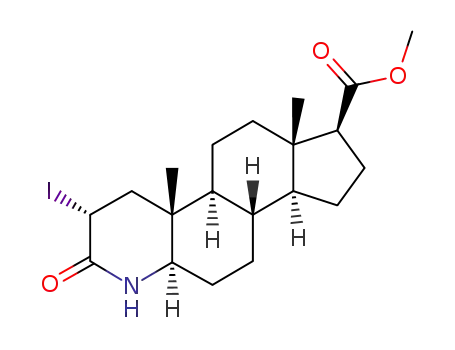
- 149198-45-8
methyl (2α,5α,17β)-2-iodo-3-oxo-4-aza-5-androstane-17-carboxylate

-
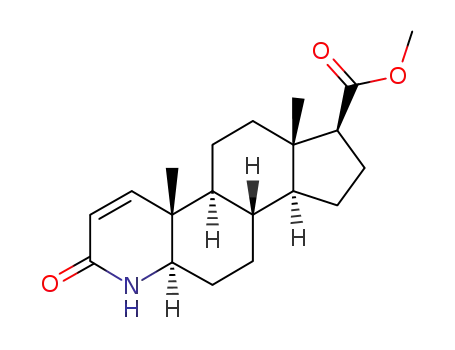
- 103335-41-7
methyl 3-oxo-4-aza-5α-androst-1-ene-17β-carboxylate
| Conditions | Yield |
|---|---|
|
With Oxone; sodium hydrogencarbonate; In methanol; water; at 30 ℃; for 3h; Time;
|
91% |
-
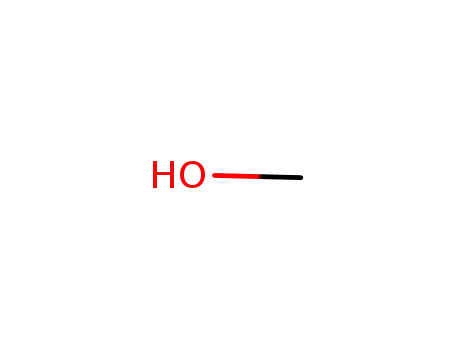
- 67-56-1
methanol

-
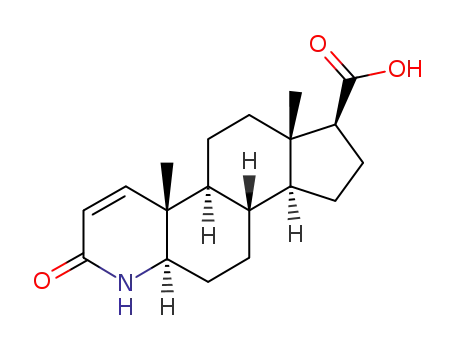
- 104239-97-6
3-Oxo-4-aza-5α-androst-1-ene-17β-carboxylic acid

-

- 103335-41-7
methyl 3-oxo-4-aza-5α-androst-1-ene-17β-carboxylate
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 20 ℃; for 14h;
|
89.35% |
103335-41-7 Upstream products
-
73671-92-8
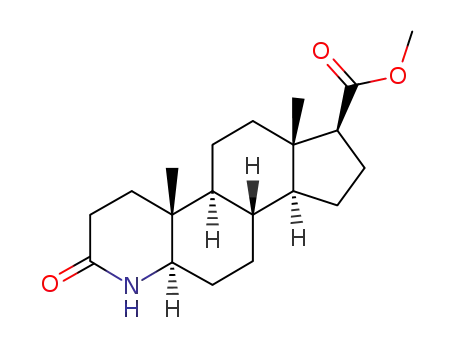
3-oxo-4-aza-5α-androstane-17β-carboxylic acid methyl ester
-
17697-12-0
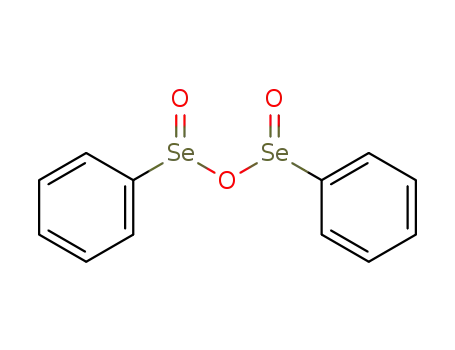
benzeneseleninic anhydride
-
149198-45-8

methyl (2α,5α,17β)-2-iodo-3-oxo-4-aza-5-androstane-17-carboxylate
-
67-56-1

methanol
103335-41-7 Downstream products
-
103335-44-0
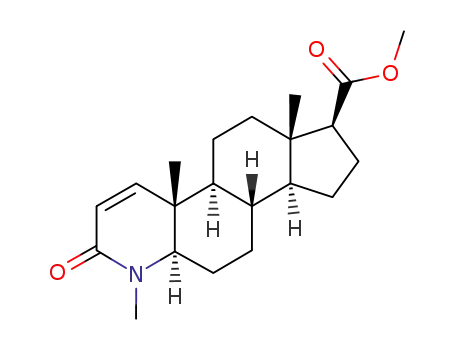
Methyl 4-methyl-3-oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide
-
1026013-15-9
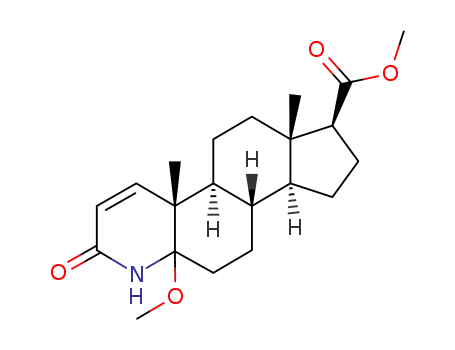
(4aR,4bS,6aS,7S,9aS,9bS)-11a-Methoxy-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxylic acid methyl ester
-
496947-19-4
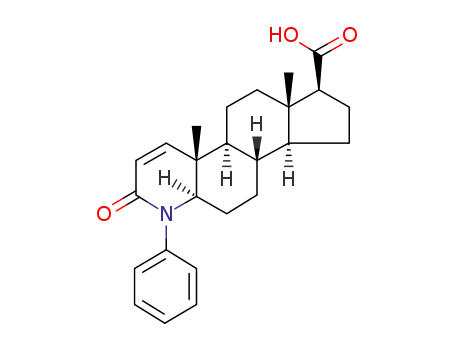
3-Oxo-4-phenyl-4-aza-5α-androst-1-ene-17β-carboxylic acid
-
98319-26-7
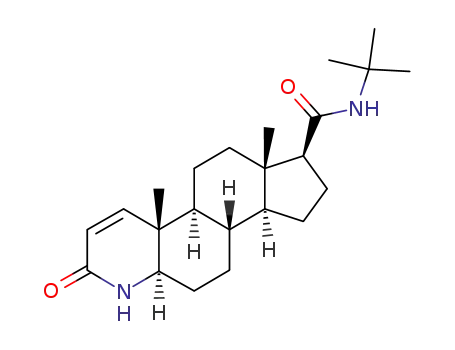
finasteride
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
Fulvestrant
CAS:129453-61-8
-
(1S,4S)-Methyl 4-aminocyclohexanecarboxylate
CAS:62456-15-9