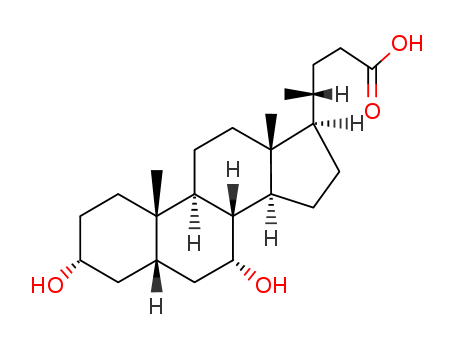
474-25-9
- Product Name:Chenodeoxycholic Acid
- Molecular Formula:C24H40O4
- Purity:99%
- Molecular Weight:392.579
Product Details;
CasNo: 474-25-9
Molecular Formula: C24H40O4
Appearance: Off-white solid
Buy Quality Sale Chenodeoxycholic Acid 474-25-9 Cheapest Price
- Molecular Formula:C24H40O4
- Molecular Weight:392.579
- Appearance/Colour:Off-white solid
- Melting Point:165-167 °C(lit.)
- Refractive Index:1.558
- Boiling Point:547.148 °C at 760 mmHg
- PKA:pKa 4.34 (Uncertain)
- Flash Point:298.8 °C
- PSA:77.76000
- Density:1.129 g/cm3
- LogP:4.47790
Chenodeoxycholic acid(Cas 474-25-9) Usage
|
Uses |
Chenodeoxycholic acid is a bile acid synthesized in the liver from cholesterol. henodeoxycholic acid has been used in a study to assess its effects as a long-term replacement therapy for cerebrotendinous xanthomatosis (CTX). It has also been used in a study to investigate its effects on the small-intestinal absorption of bile acids in patients with ileostomies. |
|
Description |
Chenodeoxycholic acid is the first agent to be introduced into the US market for the treatment of radiolucent gallstones. Large scale clinical trials have demonstrated the safety and efficacy of this agent. Chenodeoxycholic acid reduces the biliary concentration of cholesterol relative to that of bile acids and phospholipid, reducing the saturation and thus the lithogenicity of the bile. Success rates in dissolving gallstones are in the range of 50-70% within 4-24 months of treatment. Continuation of the drug after stone dissolution may be required to prevent reoccurrence. Chenodeoxycholic acid is the 7α-isomer of ursodeoxycholic acid which was introduced into the European market in 1978. chenodeoxycholic acid structure |
|
Chemical Properties |
Off-White Solid |
|
Originator |
Rowell (USA) |
|
History |
Chenodeoxycholic acid was isolated in 1924 from goose gall by Adolf Windaus and human gall by Heinrich Wieland.Its complete structural configuation was elucidated by Hans Lettre at the University of Gottingen. In 1968, William Admirand and Donald Small at Boston University Medical School established that in patients with gallstones their bile was saturated with cholesterol, sometimes even exhibiting microcrystals, whereas this was not the case in normal people.It was then found that biliary levels of cholic acid and chenodeoxycholic acid were lower in patients with cholesterol gallstones than in normal people. Leslie Thistle and John Schoenfield at the Mayo Clinic in Rochester, Minnesota, then administered individual bile salts by mouth for four months and found that chenodeoxycholic acid reduced the amount of cholesterol in the bile.This led to a national collaborative study in the United States, which confirmed the effectiveness of chenodeoxycholic acid in bringing about dissolution of gallstones in selected patients. However, recent developments such as laparoscopic cholecystectomy and endoscopic biliary techniques have curtailed the role of chenodeoxycholic acid and ursodeoxycholic acid in the treatment of cholelithiasis. |
|
Manufacturing Process |
To 1,400 ml of an approximately 50% water/triglycol solution of the potassium salt of chenodeoxycholic acid, obtained by the Wolff-Kishner reduction (using hydrazine hydrate and potassium hydroxide) from 50 g of 7- acetyl-12-ketochenodeoxycholic acid, 220 ml of dilute hydrochloric acid is added to bring the pH to 2. The solution is stirred and the crude chenodeoxycholic acid precipitates. The precipitate is recovered and dried to constant weight at about 60°C. About 36 g of the crude chenodeoxycholic acid, melting in the range of 126°-129°C, is obtained. 25 g of crude chenodeoxycholic acid so obtained is dissolved in 750 ml of acetonitrile while stirring and heating. 3 g of activated charcoal is added and then removed by suction filtering. The resulting liquid filtrate is cooled, the pure chenodeoxycholic acid crystallizing out. The crystals are recovered by suction filtering and the recovered crystals dried under vacuum. The yield is 19 g of pure chenodeoxycholic acid with a melting range of 168°-171°C. |
|
Brand name |
CHEWM |
|
Therapeutic Function |
Gallostone dissolving agent |
|
World Health Organization (WHO) |
Chenodeoxycholic acid was introduced in 1975 for the treatment of cholelithiasis. It is available in several countries and the World Health Organization is not aware that registration has been refused in any other country. |
|
General Description |
Chenodeoxycholic acid is a bile acid synthesized in the liver from cholesterol. |
|
Flammability and Explosibility |
Nonflammable |
|
Purification Methods |
This major bile acid in vertebrates (~80mg) is chromatographed on silica gel (5g) and eluted with CHCl3/EtOAc (3:2) and crystallised from EtOAc/hexane. It has IR: max 1705 cm-1(CHCl3). It also crystallises from EtOAc, EtOAc/heptane after purifying via the poorly soluble Na and K salt if necessary. [Kametani et al. J Org Chem 4 7 2331 1982, Beilstein 10 IV 1604.] |
InChI:InChI=1/C24H40O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-20,22,25-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16-,17+,18+,19-,20+,22?,23+,24-/m1/s1
474-25-9 Relevant articles
Solvolysis of chenodeoxycholic acid sulfates
Cohen,Budai,Javitt
, p. 621 - 626 (1981)
-
Non-stereospecific formation of 3α,7α,24-trihydroxy-5β-cholestan-26- oic acid during chenodeoxycholic acid biosynthesis
Kobayashi,Hagiwara,Morisaki,Yuri,Fujimoto
, p. 1536 - 1538 (1994)
Incubation of 3α,7α-dihydroxy-5β-cholest...
Engineering Regioselectivity of a P450 Monooxygenase Enables the Synthesis of Ursodeoxycholic Acid via 7β-Hydroxylation of Lithocholic Acid
Grobe, Sascha,Badenhorst, Christoffel P. S.,Bayer, Thomas,Hamnevik, Emil,Wu, Shuke,Grathwol, Christoph W.,Link, Andreas,Koban, Sven,Brundiek, Henrike,Gro?johann, Beatrice,Bornscheuer, Uwe T.
, p. 753 - 757 (2020/12/01)
We engineered the cytochrome P450 monoox...
Method for synthesizing 3alpha, 7alpha-dihydroxy-5-beta-cholanic acid from duck cholic acid
-
Paragraph 0013, (2021/02/06)
The invention belongs to the field of or...
Preparation method of chenodeoxycholic acid
-
Paragraph 0037; 0061-0063, (2021/05/01)
The invention belongs to the field of or...
Preparation method of chenodeoxycholic acid
-
Paragraph 0089-0090; 0138-0143; 0181-0183, (2021/01/24)
The invention relates to the technical f...
474-25-9 Process route
-
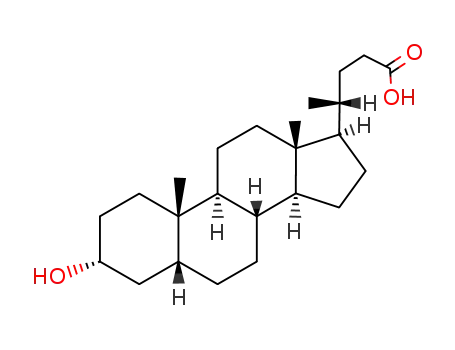
- 434-13-9
Lithocholic acid

-
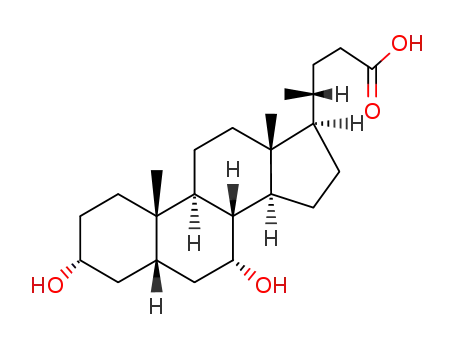
- 474-25-9
chenodeoxycholic acid

-
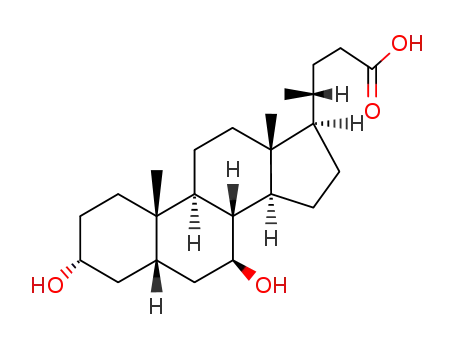
- 128-13-2
ursodeoxycholic acid

-
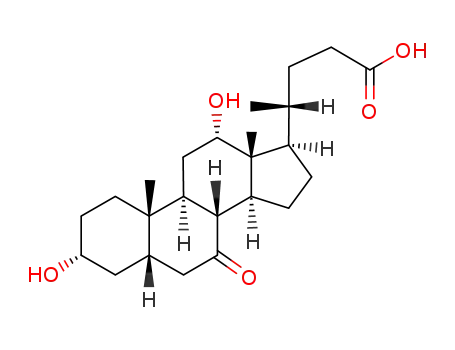
- 911-40-0
7-ketodeoxycholic acid
| Conditions | Yield |
|---|---|
|
With Lentzea waywayandensis VKM Ac-1970; In methanol; aq. phosphate buffer; pH=7; Microbiological reaction;
|
-
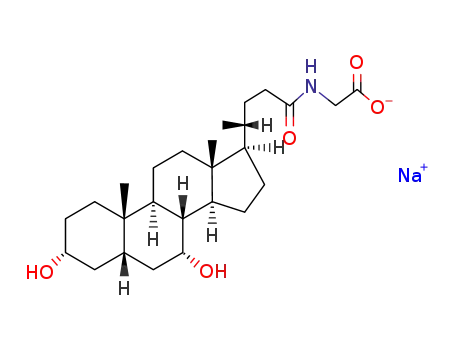
- 16564-43-5
glycochenodeoxycholic acid sodium salt

-

- 474-25-9
chenodeoxycholic acid

-
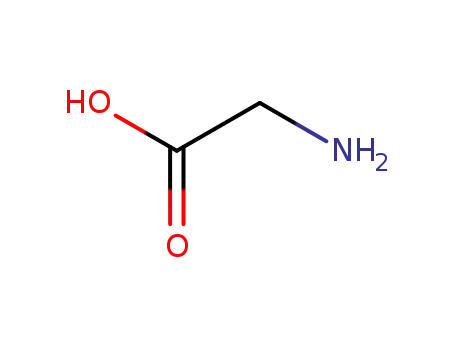
- 56-40-6,18875-39-3,25718-94-9
glycine
| Conditions | Yield |
|---|---|
|
With ethylenediaminetetraacetic acid; chloylglycine hydrolase; 2-hydroxyethanethiol; In phosphate buffer; at 20 ℃; for 3h; pH=8;
|
100 % Chromat. |
474-25-9 Upstream products
-
859-97-2
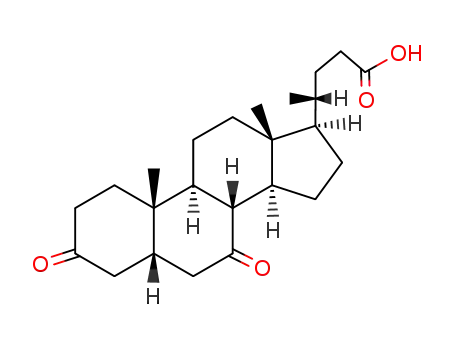
3,7-diketocholanic acid
-
28535-81-1
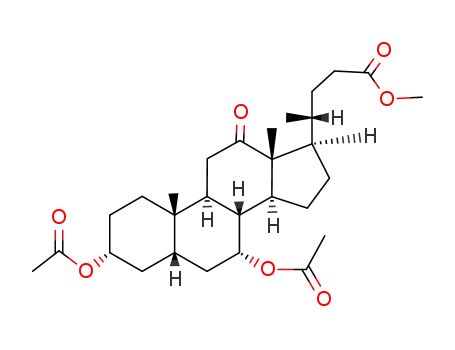
methyl 3α,7α-diacetoxy-12-one-5β-cholest-24-carboxylate
-
4651-67-6
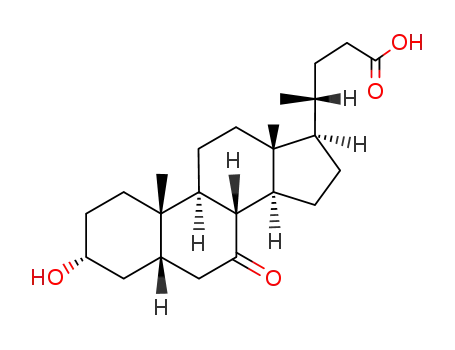
7-Ketolithocholic acid
-
141-52-6
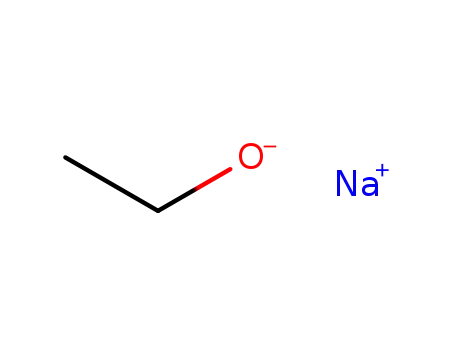
sodium ethanolate
474-25-9 Downstream products
-
88165-77-9
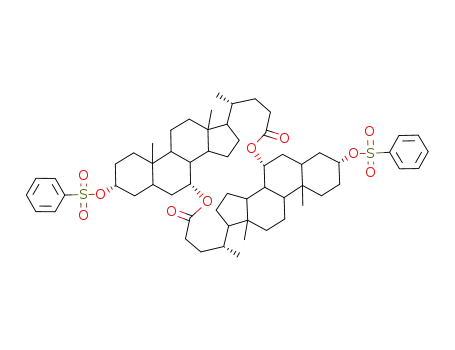
bis(3α-phenylsulfonyloxy-5β-cholansaeure-7α-yl)diester
-
92054-20-1
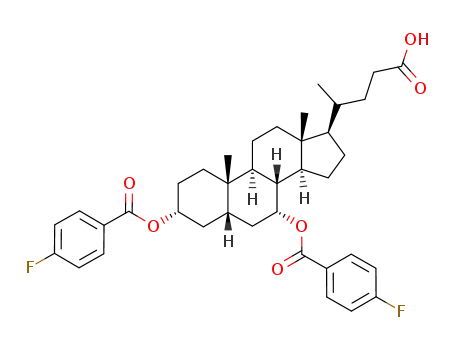
5β-cholanic acid-3α,7α-diol di-p-fluorobenzoate
-
3862-26-8
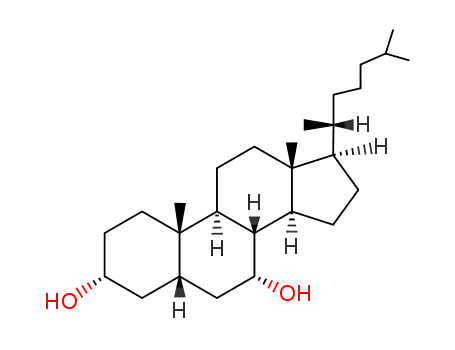
5β-cholestane-3α,7α-diol
-
4651-67-6

7-Ketolithocholic acid
Relevant Products
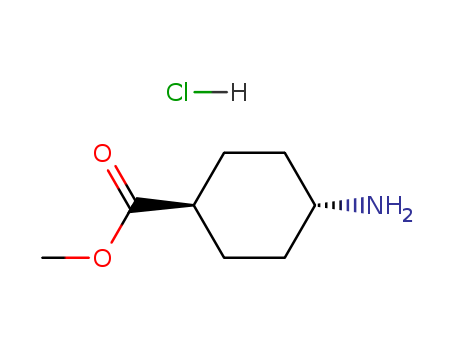
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
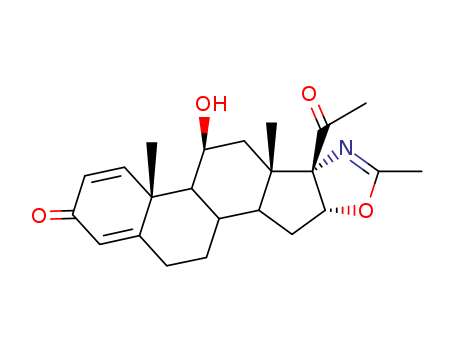
-
Deflazacort intermediate(D5)
CAS:13649-88-2
-
16alpha-Hydroxyprednisolone
CAS:13951-70-7