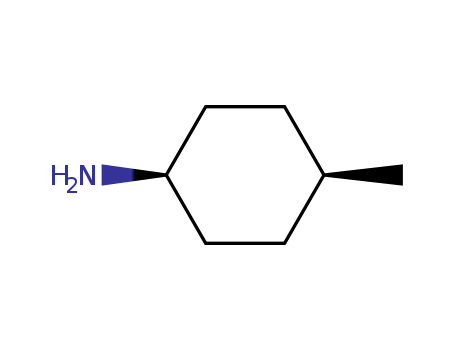
148849-67-6
- Product Name:Ivabradine Hydrochloride
- Molecular Formula:C27H36N2O5.HCl
- Purity:99%
- Molecular Weight:505.054
Product Details;
CasNo: 148849-67-6
Molecular Formula: C27H36N2O5.HCl
Buy High Quality Top Purity 99% Ivabradine Hydrochloride 148849-67-6 Safe Delivery
- Molecular Formula:C27H36N2O5.HCl
- Molecular Weight:505.054
- Vapor Pressure:1.24E-15mmHg at 25°C
- Melting Point:193-196?C
- Boiling Point:626.9 °C at 760 mmHg
- Flash Point:332.9 °C
- PSA:60.47000
- LogP:4.04990
Ivabradine hydrochloride(Cas 148849-67-6) Usage
|
Description |
In an effort to develop angina agents without the unwanted negative inotropic and hypotensive effects associated with b-adrenergic blockers and calcium channel blockers, a new class of heart-rate reducing compounds that act specifically on the sinoatrial (SA) node has been explored. These bradycardic agents interact directly with the pacemaking cell of the SA node and the hyperpolarization- activated If , the primary pacemaking current. Ivabradine has evolved as a specific inhibitor of If current through its contact with f-channels on the intracellular side of the plasma membrane. As a consequence, ivabradine reduces the speed of diastolic depolarization and decreases heart rate. It has been approved for the treatment of chronic stable angina and provides a viable alternative to patients with a contraindication or intolerance of b-blockers. Evaluation is also underway for the potential treatment of ischemic heart disease. Using a patch-clamp technique on rabbit sinoatrial node cells, inhibition of If current ranged from 6% (0.03 mM) – 80% (10 mM). . |
|
Chemical Properties |
White to Off-White Solid |
|
Originator |
Servier (France) |
|
Uses |
Ivabradine hydrochloride has been used as a potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel (HCN)2 blocker in embryoid body (EB) and rat engineered heart tissue (EHT). |
|
Definition |
ChEBI: A hydrochloride obtained by combining ivabradine with one molar equivalent of hydrochloric acid. Used to treat patients with angina who have intolerance to beta blockers and/or heart failure. |
|
Brand name |
Procoralan |
|
Biochem/physiol Actions |
Ivabradine is used to treat chronic heart failure. |
|
Clinical Use |
Symptomatic treatment of chronic stable angina pectoris in patients with sinus rhythm Treatment of mild to severe chronic heart failure |
|
Drug interactions |
Potentially hazardous interactions with other drugs Anti-arrhythmics: increased risk of ventricular arrhythmias with amiodarone and disopyramide. Antibacterials: concentration possibly increased by clarithromycin and telithromycin - avoid; increased risk of ventricular arrhythmias with erythromycin - avoid. Antifungals: concentration increased by ketoconazole - avoid; concentration increased by fluconazole - reduce initial ivabradine dose; concentration possibly increased by itraconazole - avoid. Antimalarials: increased risk of ventricular arrhythmias with mefloquine. Antipsychotics: increased risk of ventricular arrhythmias with pimozide. Antivirals: concentration possibly increased by ritonavir - avoid. Beta-blockers: increased risk of ventricular arrhythmias with sotalol. Calcium-channel blockers: concentration increased by diltiazem and verapamil - avoid. Grapefruit juice: ivabradine concentration increased. Pentamidine: increased risk of ventricular arrhythmias. St John’s wort: ivabradine concentration reduced - avoid. |
|
Metabolism |
Ivabradine is extensively metabolised by the liver and the gut by oxidation through cytochrome P450 3A4 (CYP3A4) only. The major active metabolite is N-desmethyl-ivabradine (S 18982) with an exposure about 40% of that of the parent compound. This active metabolite undergoes further metabolism by CYP3A4. Excretion of metabolites occurs to a similar extent via faeces and urine. |
InChI:InChI=1/C27H36N2O5.ClH/c1-28(17-21-11-20-14-25(33-4)26(34-5)16-22(20)21)8-6-9-29-10-7-18-12-23(31-2)24(32-3)13-19(18)15-27(29)30;/h12-14,16,21H,6-11,15,17H2,1-5H3;1H/t21-;/m1./s1
148849-67-6 Relevant articles
AN IMPROVED PROCESS FOR THE SYNTHESIS OF IVABRADINE AND ITS PHARMACEUTICALLY ACCEPTABLE SALTS
-
, (2019/11/12)
: Disclosed herein is an improved proces...
Preparation method of high-purity ivabradine hydrochloride and intermediate thereof
-
Paragraph 0056-0062, (2019/12/02)
The invention discloses a preparation me...
(1 S) - 4, 5 - dimethoxy - 1 - [(methylamino) methyl] benzocyclobutane preparation of hydrochloride salts of method
-
, (2018/04/02)
The invention provides a preparation met...
A high-purity hydrochloric acid Ivabradine preparation method (by machine translation)
-
Paragraph 0057; 0059-0062; 0063-0069, (2018/09/11)
The invention discloses a high-purity hy...
148849-67-6 Process route
-
![3-(2-[1,3]-dioxolan-2-yl-ethyl)-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one](/upload/2023/9/6da8d0fd-bcd7-4ad8-9f52-d032454c2a08.png)
-
3-(2-[1,3]-dioxolan-2-yl-ethyl)-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one

-
![1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride](/upload/2023/9/7ec2751a-67a3-46ca-9a5b-f4cd10b02ddb.png)
- 866783-13-3
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride

-
- 148849-67-6,148849-68-7
ivabradine hydrochloride
| Conditions | Yield |
|---|---|
|
3-(2-[1,3]-dioxolan-2-yl-ethyl)-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one; With hydrogen; palladium on activated charcoal; In ethanol; at 55 ℃; under 3750.38 Torr;
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride; In ethanol; water; at 20 - 85 ℃; under 22502.3 Torr;
|
85% |
|
3-(2-[1,3]-dioxolan-2-yl-ethyl)-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one; With palladium on activated charcoal; hydrogen; In ethanol; at 55 ℃; under 3750.38 Torr; Autoclave; Large scale;
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride; With palladium on activated charcoal; hydrogen; In ethanol; water; at 85 ℃; under 22502.3 Torr; Autoclave; Large scale;
|
85% |
|
3-(2-[1,3]-dioxolan-2-yl-ethyl)-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one; With palladium on activated charcoal; hydrogen; In ethanol; at 20 - 55 ℃; under 3750380 Torr; Autoclave; Inert atmosphere;
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride; In ethanol; water; at 20 - 85 ℃; under 63756400 Torr; Inert atmosphere;
|
85% |
-
![3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one](/upload/2023/9/4ef3d8f3-cff1-4e02-9411-cc5f19a172f2.png)
- 1086026-31-4
3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one

-

- 148849-67-6,148849-68-7
ivabradine hydrochloride
| Conditions | Yield |
|---|---|
|
3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one; With palladium 10% on activated carbon; hydrogen; acetic acid; at 15 - 25 ℃; for 23h;
With hydrogenchloride; In ethyl acetate; isopropyl alcohol; at 0 - 10 ℃; for 1h;
|
93.5% |
|
3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one; With palladium 10% on activated carbon; hydrogen; ammonium formate; In methanol; at 25 - 30 ℃;
With hydrogenchloride; In dichloromethane;
|
78% |
|
With hydrogenchloride; hydrogen; palladium 10% on activated carbon; In water; at 45 ℃; for 6h; under 7500.75 Torr;
|
|
|
Multi-step reaction with 2 steps
1: hydrogenchloride / acetonitrile / 20 - 25 °C
2: 5%-palladium/activated carbon; hydrogen / methanol / 18 h / 30 - 35 °C / 3000.3 - 3750.38 Torr / Autoclave
With hydrogenchloride; 5%-palladium/activated carbon; hydrogen; In methanol; acetonitrile;
|
|
|
3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one; With palladium 10% on activated carbon; hydrogen; In methanol; at 25 - 30 ℃; under 5250.53 - 6000.6 Torr;
With hydrogenchloride; In dichloromethane; water;
|
24.48 g |
148849-67-6 Upstream products
-
866783-13-3
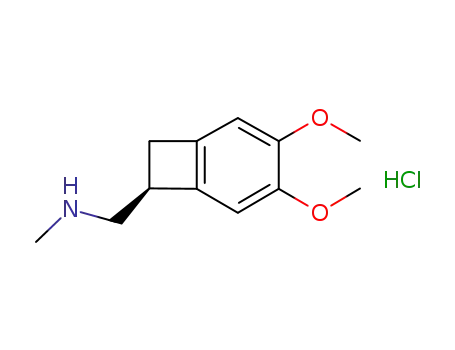
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride
-
155974-00-8
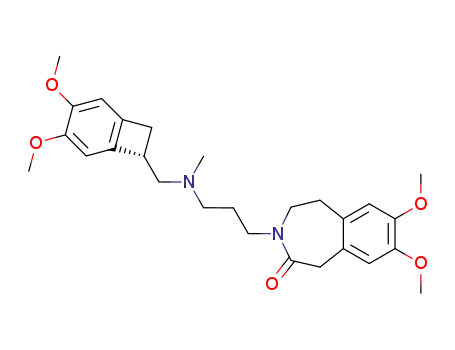
ivabradine
-
35202-54-1
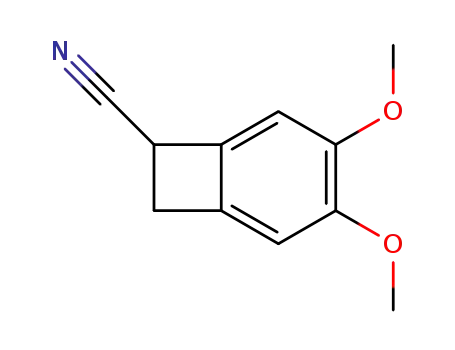
3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile
-
41234-23-5
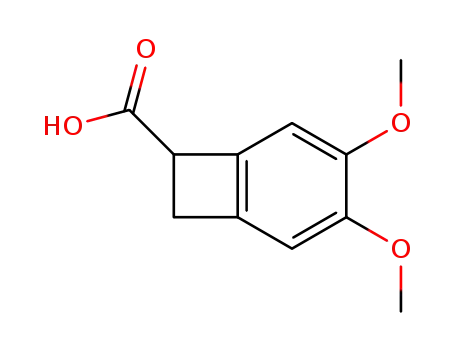
(R,S)-4,5-dimethoxy-1,2-dihydrocyclobutabenzene-1-carboxylic acid
148849-67-6 Downstream products
-
155974-00-8

ivabradine
-
1462470-54-7
{2-[2-({3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-4,3,5-trien-7-yl]methyl}(methyl)-amino]propyl}amino)ethyl]-4,5-dimethoxyphenyl}acetic acid
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
cis-4-Methylcyclohexylamine.
CAS:2523-56-0
-
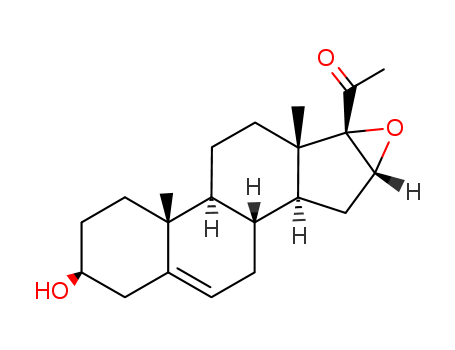
16,17a-Epoxy Pregnenolone
CAS:974-23-2