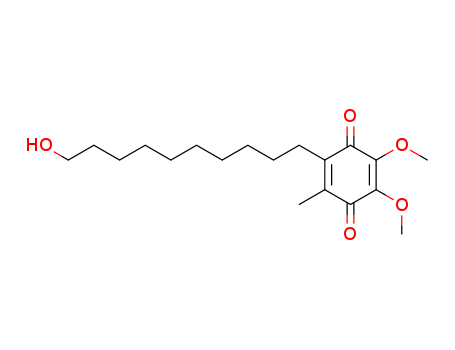
58186-27-9
- Product Name:Idebenone
- Molecular Formula:C19H30O5
- Purity:99%
- Molecular Weight:338.444
Product Details;
CasNo: 58186-27-9
Molecular Formula: C19H30O5
Appearance: Yellow-orange crystalline powder
Factory Supply High Purity High Purity Idebenone 58186-27-9 Cheap Price
- Molecular Formula:C19H30O5
- Molecular Weight:338.444
- Appearance/Colour:Yellow-orange crystalline powder
- Vapor Pressure:5.67E-12mmHg at 25°C
- Melting Point:52-55 °C
- Refractive Index:1.502
- Boiling Point:497.3 °C at 760 mmHg
- PKA:15.20±0.10(Predicted)
- Flash Point:170.1 °C
- PSA:72.83000
- Density:1.08 g/cm3
- LogP:3.46230
Idebenone(Cas 58186-27-9) Usage
|
Description |
Idebenone is an organic compound belonging to the quinone family, being similar to coenzyme Q-10. It is a kind of drug developed by Takeda Pharmaceutical Company for the treatment of Alzheimer’s disease and some other cognitive defects. However, these have been not very much progress associated with this indication. It is now also used for the treatment of Friedreich’s ataxia with a positive effect on cardiac hypertrophy and neurological function. However, this indication is only approved in Canada, not in Europe and US. It is now under investigation on its efficacy for the treatment of Duchenne muscular dystrophy, Leber’s hereditary optic neuropathy, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) as well as primary progressive multiple sclerosis. The efficacy of this drug still demands more evidences. |
|
Chemical Properties |
Yellow-Orange Crystalline Solid, odorless. Extremely insoluble in water, easily soluble in chloroform, methanol or absolute ethanol, easily soluble in ethyl acetate, and insoluble in n-hexane. |
|
Originator |
Takeda (Japan) |
|
Uses |
idebenone is an anti-oxidant capable of protecting the skin from a variety of free-radical attacks, including the formation of secondary chemicals that negatively affect skin physiology. It is said to improve intrinsic as well as extrinsic skin damage caused by freeradical formation. Idebenone is a synthetically manufactured form of coenzyme Q10 and has a smaller molecular structure. This allows it to penetrate the skin and apparently the cellular membrane. Clinical studies demonstrate a visible improvement in photodamaged skin, reduced skin roughness and dryness, decreased fine lines and wrinkles, and increased skin hydration. In addition, idebenone helps improve hyperpigmentation because its molecular structure is similar to that of hydroquinone. Although most of the associated benefits are seen primarily in the epidermis, some increase in dermal collagen has also been confirmed. Idebenone has been used for such health-related problems as Alzheimer’s and heart disease. |
|
Definition |
ChEBI: Idebenone is a member of the class of 1,4-benzoquinones which is substituted by methoxy groups at positions 2 and 3, by a methyl group at positions 5, and by a 10-hydroxydecyl group at positions 6. Initially developed for the treatment of Alzheimer's disease, benefits were modest; it was subsequently found to be of benefit for the symptomatic treatment of Friedreich's ataxia. It has a role as an antioxidant and a ferroptosis inhibitor. It is a primary alcohol and a member of 1,4-benzoquinones. |
|
Application |
Idebenone is ubiquinone derivative with protective effects against cerebral ischemia and cognition enhancer. It has been used:chemotherapy drugto treat mutant myocilin (mMYOC) cells for drug treatment assayto validate C2C12 secondary cell screening assayto treat obese mice, to test whether idebenone and CoQ10 bind directly to peroxisome proliferator-activated receptor (PPAR)LBDs, His-tagged PPARα, δ and γ LBDs |
|
Brand name |
AVAN |
|
General Description |
Idebenone is a short-chain benzoquinone drug. It is a structural relative of ubiquinone (Coenzyme Q10) that protects the CNS from the effects of ischemia via improving brain metabolism. It is reported to be of potential use in the management of patients with cerebral apoplexy, cerebral arteriosclerosis and related disorders. |
|
Biological Activity |
Antioxidant and neuroprotective agent. Protects mitochondrial membranes against lipid peroxidation and blocks glutamate neurotoxicity in vitro and in vivo . Inhibits apoptosis of astrocytes via increased NGF production. |
|
Biochem/physiol Actions |
Idebenone is used in the treatment of visual impairment in adolescents and adults with Leber′s hereditary optic neuropathy (LHON). It serves as an antioxidant. It helps to guard the heart muscle against oxidative stress. A short-chain Coenzyme Q analog that enhances superoxide formation, presumably by mediating electron transfer from N2 to oxygen. |
InChI:InChI=1/C19H30O5/c1-14-15(12-10-8-6-4-5-7-9-11-13-20)17(22)19(24-3)18(23-2)16(14)21/h20H,4-13H2,1-3H3
58186-27-9 Relevant articles
AgNO3-catalyzed decarboxylative cross-coupling reaction: an approach to coenzyme Q
Luo, Wan-Yue,Lu, Bin,Qiu, Yong-Fu,Zhou, Rong-Ye,He, Yong-Jing,Wang, Jin
, p. 8702 - 8704 (2020)
An efficient and general method for the ...
A Simple and Convenient Two-step Synthesis of Idebenone
Zhou, Rong-Ye,Li, Na,Luo, Wan-Yue,Wang, Li-Li,Zhang, Ye-Yu,Wang, Jin
, p. 397 - 401 (2021)
-
Coenzyme Q compound synthesis method
-
Paragraph 0037, (2020/11/01)
The invention relates to a coenzyme Q co...
COMPOUND CONTAINING INDOLEACETIC ACID CORE STRUCTURE AND USE THEREOF
-
, (2017/12/31)
Disclosed are a compound as shown by the...
58186-27-9 Process route

-
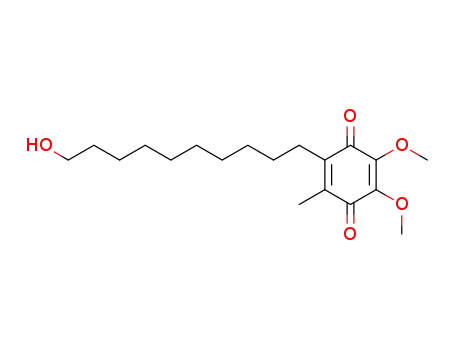
- 58186-27-9
Idebenone

-
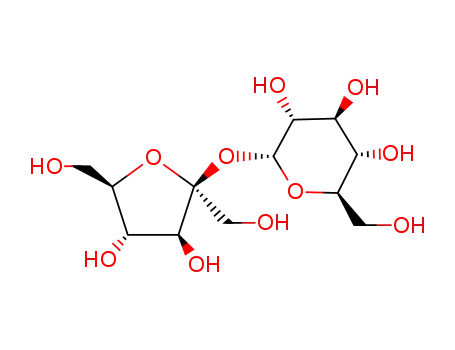
- 57-50-1
Sucrose
| Conditions | Yield |
|---|---|
|
|
-
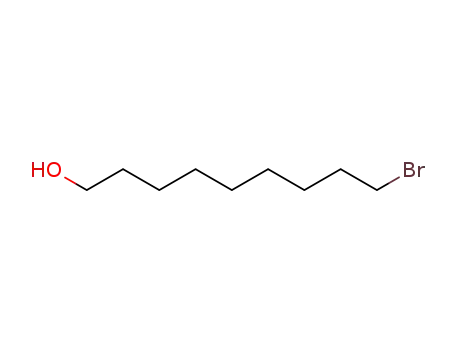
- 55362-80-6
9-bromononan-1-ol

-

- 58186-27-9
Idebenone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 9 steps
2: 95 percent / dimethylsulfoxide / 15 h / 80 °C
3: 86 percent / NaOH, 3N HCl / ethanol / 19 h / Heating
4: PCl5 / CH2Cl2 / 2 h / 25 °C
5: 44 percent / AlCl3 / 1,2-dichloro-ethane / 72 h / 20 °C
6: 79 percent / NaOH / methanol / 2 h / Ambient temperature
7: H2, 70percent HClO4 / 5percent Pd-C / 24 h / Ambient temperature
8: NaOH / methanol / 24 h / 5 °C
9: O2 / salcomine / dimethylformamide / Ambient temperature; reaction time, additive
With hydrogenchloride; sodium hydroxide; aluminium trichloride; perchloric acid; phosphorus pentachloride; hydrogen; oxygen; palladium on activated charcoal; salcomine; In methanol; ethanol; dichloromethane; dimethyl sulfoxide; 1,2-dichloro-ethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 9 steps
2: 95 percent / dimethylsulfoxide / 15 h / 80 °C
3: 86 percent / NaOH, 3N HCl / ethanol / 19 h / Heating
4: PCl5 / CH2Cl2 / 2 h / 25 °C
5: 44 percent / AlCl3 / 1,2-dichloro-ethane / 72 h / 20 °C
6: 79 percent / NaOH / methanol / 2 h / Ambient temperature
7: H2, 70percent HClO4 / 5percent Pd-C / 24 h / Ambient temperature
8: NaOH / methanol / 24 h / 5 °C
9: O2 / salcomine / dimethylformamide / Ambient temperature; reaction time, additive
With hydrogenchloride; sodium hydroxide; aluminium trichloride; perchloric acid; phosphorus pentachloride; hydrogen; oxygen; palladium on activated charcoal; salcomine; In methanol; ethanol; dichloromethane; dimethyl sulfoxide; 1,2-dichloro-ethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 9 steps
2: 95 percent / dimethylsulfoxide / 15 h / 80 °C
3: 86 percent / NaOH, 3N HCl / ethanol / 19 h / Heating
4: PCl5 / CH2Cl2 / 2 h / 25 °C
5: AlCl3 / 1,2-dichloro-ethane / 5 degC, 2 h; 20 degC, 70 h
6: 79 percent / NaOH / methanol / 2 h / Ambient temperature
7: H2, 70percent HClO4 / 5percent Pd-C / 24 h / Ambient temperature
8: NaOH / methanol / 24 h / 5 °C
9: O2 / salcomine / dimethylformamide / Ambient temperature; reaction time, additive
With hydrogenchloride; sodium hydroxide; aluminium trichloride; perchloric acid; phosphorus pentachloride; hydrogen; oxygen; palladium on activated charcoal; salcomine; In methanol; ethanol; dichloromethane; dimethyl sulfoxide; 1,2-dichloro-ethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 9 steps
2: 95 percent / dimethylsulfoxide / 15 h / 80 °C
3: 86 percent / NaOH, 3N HCl / ethanol / 19 h / Heating
4: PCl5 / CH2Cl2 / 2 h / 25 °C
5: 29 percent / AlCl3 / 1,2-dichloro-ethane / 72 h / 20 °C
6: 79 percent / NaOH / methanol / 2 h / Ambient temperature
7: H2, 70percent HClO4 / 5percent Pd-C / 24 h / Ambient temperature
8: NaOH / methanol / 24 h / 5 °C
9: O2 / salcomine / dimethylformamide / Ambient temperature; reaction time, additive
With hydrogenchloride; sodium hydroxide; aluminium trichloride; perchloric acid; phosphorus pentachloride; hydrogen; oxygen; palladium on activated charcoal; salcomine; In methanol; ethanol; dichloromethane; dimethyl sulfoxide; 1,2-dichloro-ethane; N,N-dimethyl-formamide;
|
58186-27-9 Upstream products
-
58186-02-0
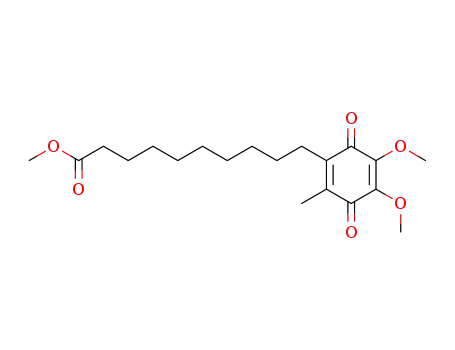
2,3-dimethoxy-5-methyl-6-(9'-methoxycarbonylnonyl)-1,4-benzoquinone
-
77712-29-9
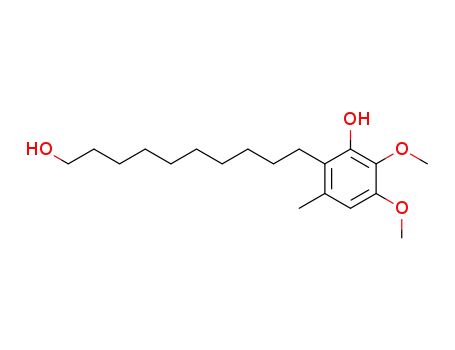
6-(10-hydroxydecyl)-2,3-dimethoxy-5-methylphenol
-
58186-28-0
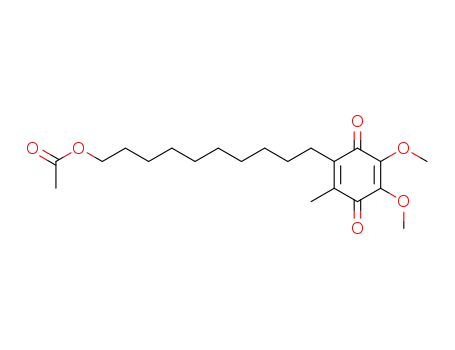
2,3-dimethoxy-5-methyl-6-(10'-acetoxydecyl)-1,4-benzoquinone
-
14065-32-8
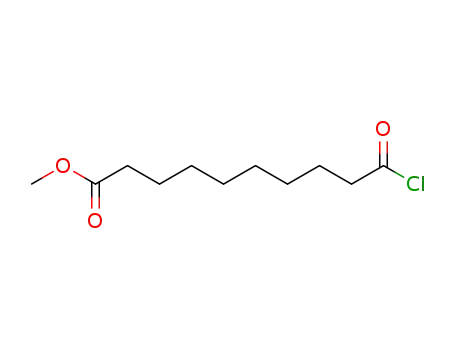
methyl 10-chloro-10-oxodecanoate
58186-27-9 Downstream products
-
58185-99-2
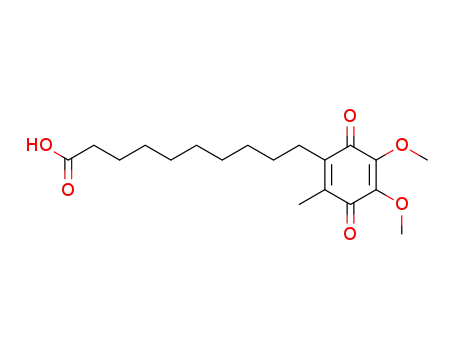
10-(2,3-Dimethoxy-5-methyl-1,4-benzoquinon-6-yl)decanoic acid
-
116460-82-3
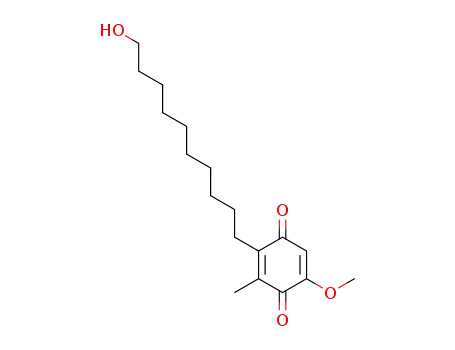
6-(10-hydroxydecyl)-3-methoxy-5-methyl-1,4-benzoquinone
-
116460-81-2

6-(10-hydroxydecyl)-2-methoxy-5-methyl-1,4-benzoquinone
-
88543-33-3
2-hydroxy-6-(10-hydroxydecyl)-3-methoxy-5-methyl-1,4-benzoquinone
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
Altrenogest
CAS:850-52-2
-
2-Methyl-1,3-cyclopentanedione
CAS:765-69-5