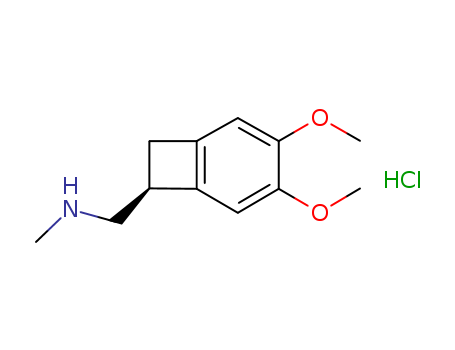
866783-13-3
- Product Name:(7S)-3,4-dimethoxy-N-methyl-Bicyclo[4.2.0]octa-1,3,5-triene-7-methanamine hydrochloride
- Molecular Formula:C12H18ClNO2
- Purity:99%
- Molecular Weight:243.733
Product Details;
CasNo: 866783-13-3
Molecular Formula: C12H18ClNO2
Factory Supply High Purity (7S)-3,4-dimethoxy-N-methyl-Bicyclo[4.2.0]octa-1,3,5-triene-7-methanamine hydrochloride 866783-13-3 Fast Shipping
- Molecular Formula:C12H18ClNO2
- Molecular Weight:243.733
- Vapor Pressure:6.95E-05mmHg at 25°C
- Boiling Point:338.6 °C at 760 mmHg
- Flash Point:158.6 °C
- PSA:30.49000
- LogP:2.75580
(1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride(Cas 866783-13-3) Usage
|
Uses |
(1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride is an intermediate used in preparation of Ivabradine (I940500) which is a selective bradycardic agent with direct effect on the pacemaker If current of the sinoatrial node. |
InChI:InChI=1/C12H17NO2.ClH/c1-13-7-9-4-8-5-11(14-2)12(15-3)6-10(8)9;/h5-6,9,13H,4,7H2,1-3H3;1H/t9-;/m1./s1
866783-13-3 Relevant articles
AN IMPROVED PROCESS FOR THE SYNTHESIS OF IVABRADINE AND ITS PHARMACEUTICALLY ACCEPTABLE SALTS
-
, (2019/11/12)
: Disclosed herein is an improved proces...
(1 S) - 4, 5 - dimethoxy - 1 - [(methylamino) methyl] benzocyclobutane preparation of hydrochloride salts of method
-
Paragraph 0109; 0116; 0121; 0122, (2018/04/02)
The invention provides a preparation met...
Exploiting the Biocatalytic Toolbox for the Asymmetric Synthesis of the Heart-Rate Reducing Agent Ivabradine
Pedragosa-Moreau, Sandrine,Le Flohic, Alexandre,Thienpondt, Vivien,Lefoulon, Fran?ois,Petit, Anne-Marie,Ríos-Lombardía, Nicolás,Morís, Francisco,González-Sabín, Javier
supporting information, p. 485 - 493 (2017/02/10)
Several chemoenzymatic routes have been ...
Process for the enzymatic synthesis of (7S)-3,4-dimethoxybicyclo[4.2.0]OCTA-1,3,5-triene-7-carboxylic acid and application in the synthesis of ivabradine and salts thereof
-
, (2016/11/14)
Process for the enzymatic synthesis of t...
866783-13-3 Process route
-
![[{(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl}methyl](methyl)amine](/upload/2023/9/606e4867-2ba4-4060-aec6-44c6939aa4ac.png)
-
866783-12-2
[{(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl}methyl](methyl)amine

-
![1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride](/upload/2023/9/b2c96dba-cfe5-4968-a8dd-26d210d5703b.png)
-
866783-13-3
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride
| Conditions | Yield |
|---|---|
|
[{(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl}methyl](methyl)amine;
With
acetic acid;
In
ethanol;
at 20 ℃;
for 0.5h;
With
hydrogenchloride;
at 15 - 20 ℃;
for 1h;
|
95% |
|
With
hydrogenchloride;
In
ethanol; ethyl acetate;
at 15 - 20 ℃;
for 1h;
Industry scale;
|
92% |
-
![(7S)-3,4-dimethoxy-N-methylbicyclo[4.2.0]octa-1,3,5-triene-7-carboxamide](/upload/2023/9/8d588b77-a710-404d-8efd-68644ec4f245.png)
-
1220993-43-0
(7S)-3,4-dimethoxy-N-methylbicyclo[4.2.0]octa-1,3,5-triene-7-carboxamide

-
![1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride](/upload/2023/9/b2c96dba-cfe5-4968-a8dd-26d210d5703b.png)
-
866783-13-3
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride
| Conditions | Yield |
|---|---|
|
(7S)-3,4-dimethoxy-N-methylbicyclo[4.2.0]octa-1,3,5-triene-7-carboxamide;
With
borane-THF;
In
tetrahydrofuran;
at 20 - 50 ℃;
With
methanol;
In
tetrahydrofuran;
at 0 - 5 ℃;
for 0.25h;
With
hydrogenchloride;
In
ethyl acetate;
at 0 ℃;
for 2.5h;
Reflux;
|
88% |
|
(7S)-3,4-dimethoxy-N-methylbicyclo[4.2.0]octa-1,3,5-triene-7-carboxamide;
With
borane-THF;
In
tetrahydrofuran;
at 20 ℃;
With
hydrogenchloride;
In
tetrahydrofuran; ethanol;
for 4h;
|
77% |
866783-13-3 Upstream products
-
866783-12-2
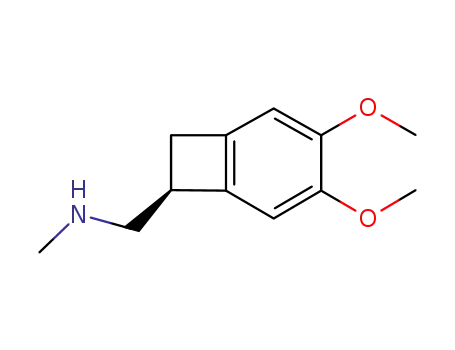
[{(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl}methyl](methyl)amine
-
35202-54-1
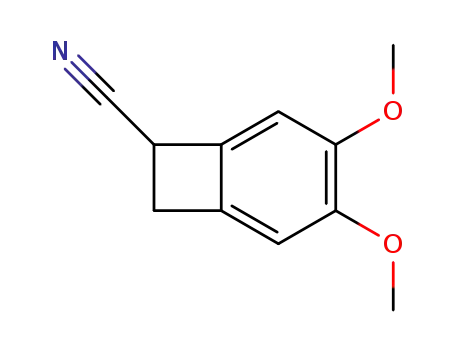
3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile
-
41234-23-5

(R,S)-4,5-dimethoxy-1,2-dihydrocyclobutabenzene-1-carboxylic acid
-
1220993-44-1
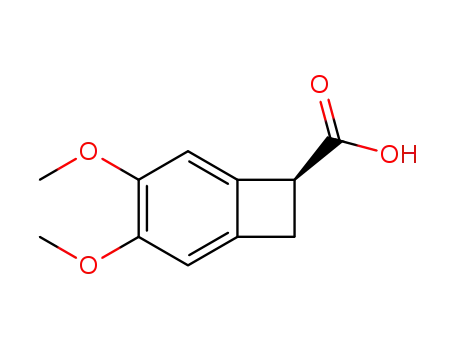
(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid
866783-13-3 Downstream products
-
148849-67-6
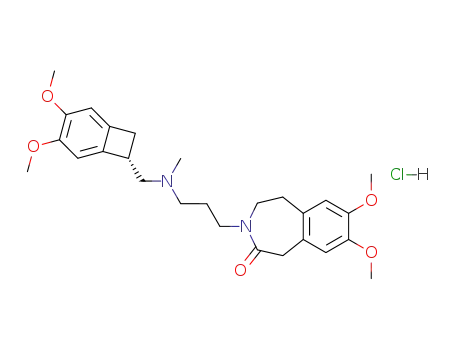
ivabradine hydrochloride
-
1086026-31-4
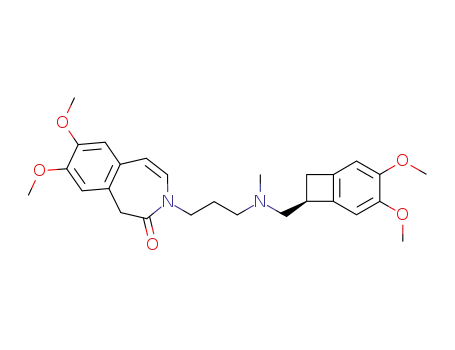
3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one
-
1462470-53-6
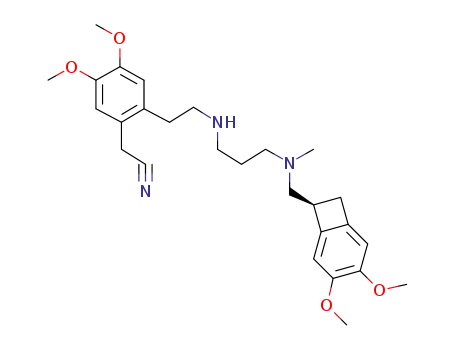
{2-[2-({3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}(methyl)-amino]propyl}amino)ethyl]-4,5-dimethoxyphenyl}acetonitrile
-
1462470-54-7
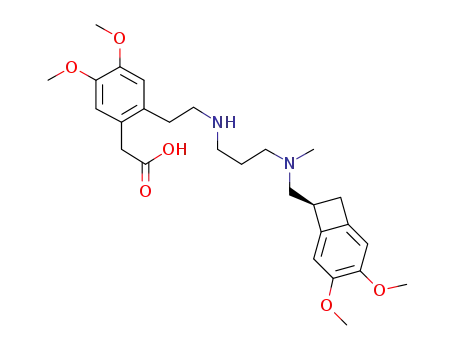
{2-[2-({3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-4,3,5-trien-7-yl]methyl}(methyl)-amino]propyl}amino)ethyl]-4,5-dimethoxyphenyl}acetic acid
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
2-Methyl-1,3-cyclopentanedione
CAS:765-69-5
-
Triamcinolone Acetonide
CAS:76-25-5