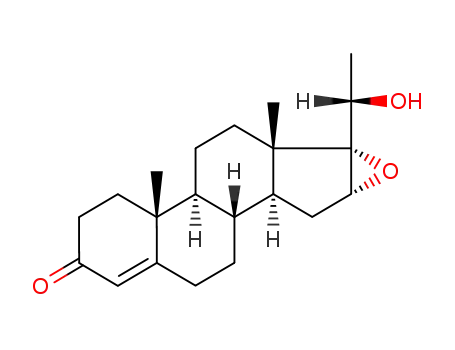
1097-51-4
- Product Name:16-17A-Epoxyprogesterone
- Molecular Formula:C21H28O3
- Purity:99%
- Molecular Weight:328.452
Product Details;
CasNo: 1097-51-4
Molecular Formula: C21H28O3
Appearance: white crystalline powder
Buy Reliable Quality Top Purity 16-17A-Epoxyprogesterone 1097-51-4 Competitive Price
- Molecular Formula:C21H28O3
- Molecular Weight:328.452
- Appearance/Colour:white crystalline powder
- Vapor Pressure:6.82E-09mmHg at 25°C
- Melting Point:128 to 132oC
- Refractive Index:168 ° (C=1, CHCl3)
- Boiling Point:466.9 °C at 760 mmHg
- Flash Point:204.4 °C
- PSA:46.67000
- Density:1.18 g/cm3
- LogP:3.85480
16a,17a-Epoxyprogesterone(Cas 1097-51-4) Usage
|
Uses |
16α,17α-Epoxyprogesterone is hydrolyzed by Rhizopus nigricans for the synthesis of many steroidal drugs. |
InChI:InChI=1/C21H28O3/c1-12(22)21-18(24-21)11-17-15-5-4-13-10-14(23)6-8-19(13,2)16(15)7-9-20(17,21)3/h10,15-18H,4-9,11H2,1-3H3
1097-51-4 Relevant articles
-
Yang,Finnegan
, p. 5845,5847 (1958)
-
-
Berg
, p. 3350 (1962)
-
-
Julian et al.
, p. 367,369 (1950)
-
fertile oxide synthesis method
-
, (2017/10/20)
The invention provides a method for synt...
New process for synthesizing steroid 3-one-4-ene
-
Paragraph 0051; 0052, (2016/10/09)
The invention discloses a new process fo...
Preparation method of betamethasone intermediate
-
Paragraph 0072; 0075, (2017/02/24)
The invention relates to a preparation m...
STEROID TRANSFORMATIONS. 187. MICROBIAL CONVERSION OF 3β-HYDROXY-5α-H-PREGNANES TO THEIR Δ4-3-KETO-9α-HYDROXY DERIVATIVES
Voishvillo, N. E.,Turuta, A. M.,Kamernitskii, A. V.,Dzhlantiashvili, N. D.,Dacheva-Spasova, V. K.
, p. 177 - 182 (2007/10/02)
-
1097-51-4 Process route
-
- 2872-76-6
20α-hydroxy-16α,17α-epoxypregn-4-en-3-one

-
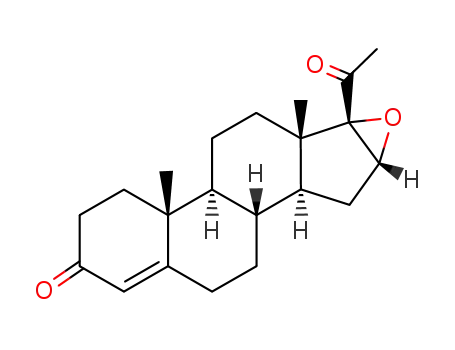
- 1097-51-4,16356-47-1
16α,17α-epoxyprogesterone
| Conditions | Yield |
|---|---|
|
With Jones reagent; In acetone; at 0 ℃; for 1h;
|
90.6% |
-
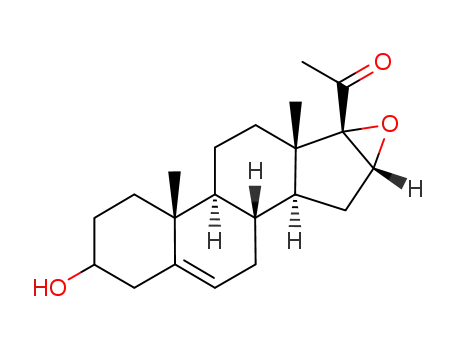
-
16α,17α-epoxy-pregnenolone

-

- 1097-51-4,16356-47-1
16α,17α-epoxyprogesterone
| Conditions | Yield |
|---|---|
|
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; ferric nitrate; In acetonitrile; for 16h; Reagent/catalyst; Reflux; Large scale;
|
100% |
1097-51-4 Upstream products
-
1096-38-4
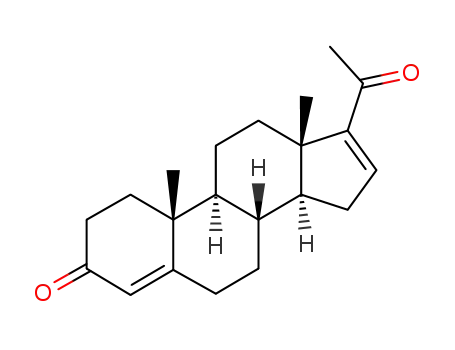
16-dehydroprogesterone
-
974-23-2
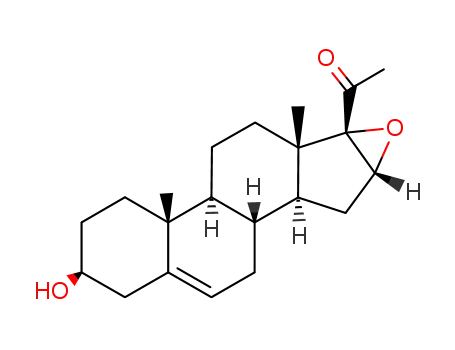
3β-hydroxy-16α,17α-epoxypregn-5-en-20-one
-
1044-90-2
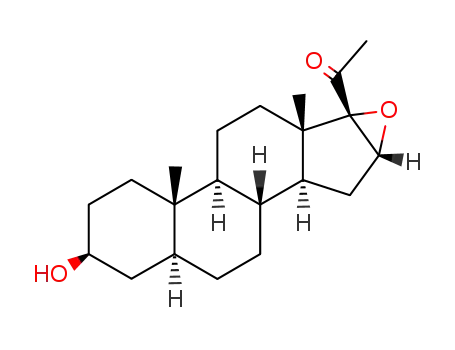
(3β,5α,16α)-16,17-epoxy-3-hydroxypregnan-20-one
-
979-02-2
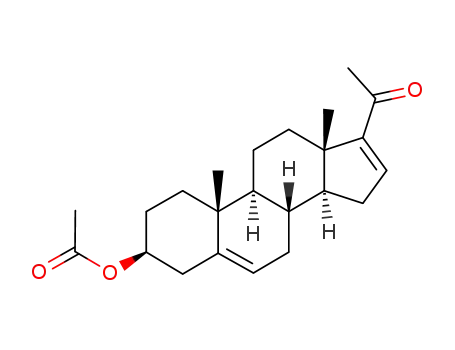
16-dehydropregnenolone acetate
1097-51-4 Downstream products
-
28018-91-9
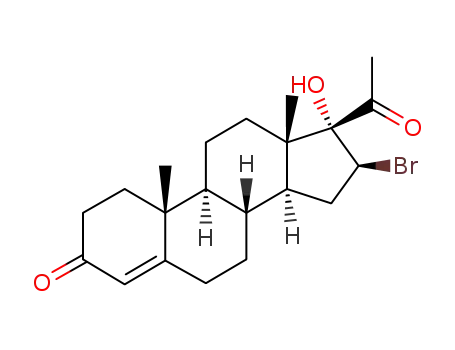
16β-Brom-17α-hydroxy-pregnen-(4)-dion-(3,20)
-
3684-83-1
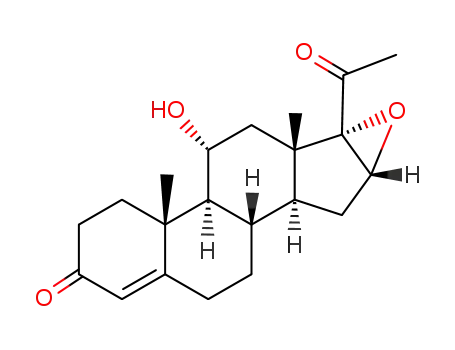
11α-hydroxy-16α,17α-epoxyprogesterone
-
152-58-9
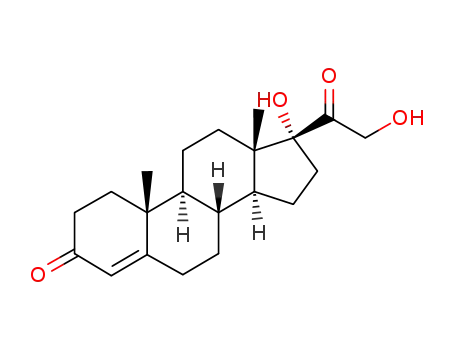
Cortexolone
-
119005-10-6
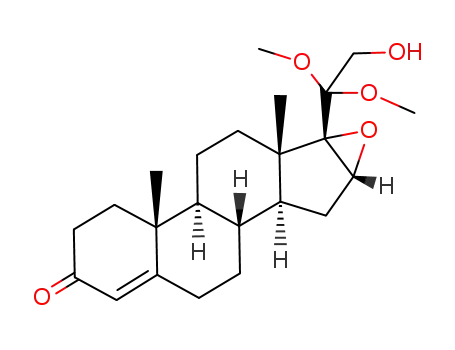
20,20-dimethoxy-16α,17α-epoxypregn-4-en-21-ol-3-one
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
Drospirenone
CAS:67392-87-4
-
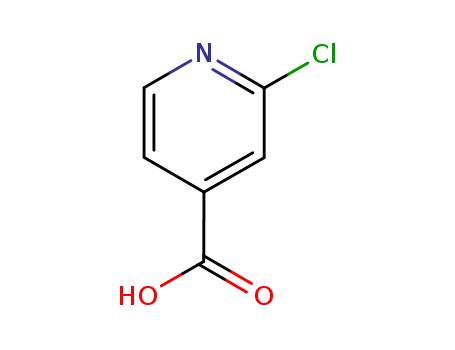
2-chloro-4-Pyridinecarboxylic aci
CAS:6313-54-8