88678-31-3
- Product Name:Liranaftate
- Molecular Formula:C18H20N2O2S
- Purity:99%
- Molecular Weight:328.435
Product Details;
CasNo: 88678-31-3
Molecular Formula: C18H20N2O2S
Appearance: white crystalline solid
Factory Supply High Purity 99% Liranaftate 88678-31-3 Safe Transportation
- Molecular Formula:C18H20N2O2S
- Molecular Weight:328.435
- Appearance/Colour:white crystalline solid
- Vapor Pressure:9.84E-09mmHg at 25°C
- Melting Point:98.5-99.5 °C
- Refractive Index:1.641
- Boiling Point:462.5 °C at 760 mmHg
- PKA:1.24±0.10(Predicted)
- Flash Point:233.5 °C
- PSA:66.68000
- Density:1.24 g/cm3
- LogP:3.76910
Liranaftate(Cas 88678-31-3) Usage
|
The third generation of dithiocarbamate antifungal |
Liranaftate is a new king of third-generation dithiocarbamate antifungals , it is a new kind of the third category of chemicals,it belongs to squalene cyclooxygenase inhibitors, by inhibiting the fungal cell membrane squalene epoxidation reaction , it hinders the synthesis of ergosterol which is a constituent of cell membranes ,it plays the role of anti-fungal,its anti-fungal activity is 8 times of tolnaftate, and its dermatophytes effect is better than clotrimazole. It has a strong anti-fungal effect on skin filamentous fungi (Trichophyton,Microsporon, Epidermophyton) , it also shows antifungal activity on other filamentous fungi, dark fungi, dimorphic fungi.It is useful in the treatment of tinea capitis, ringworm, athlete's foot, tinea pedis, onychomycosis, tinea, jock itch, tinea versicolor and vulvovaginal candidiasis. |
|
Toxicity |
Reproductive toxicity: in general reproductive toxicity tests, subcutaneous administration to SD rats , the largest non-toxic dose for the parent is 20mg/kg, the largest non-toxic dose for the reproductive process and their pups is 100mg/kg. Genetic Toxicity: Liranaftate mutagenicity tests are negative. In toxicity tests during teratogenic sensitively period , subcutaneous administration ,the largest non-toxic dose of maternal is 300mg/kg, the largest non-toxic dose to fetal rats is100mg/kg. In perinatal toxicity tests, administer subcutaneously, the largest non-toxic dose for the mother and newborn pups is 30mg/kg. |
|
Synthesis method |
5,6,7,8-tetrahydro-2-naphthol reacts with thiophosgene to give the thio-5,6,7,8-tetrahydro-2-naphthyl chloroformate ester and 2-methoxy-6-methylamine pyridine, then react in isopropanol · water at room temperature for 2 hours to obtain liranaftate ,the overall yield is 70%. |
|
Liranaftate cream |
Liranaftate cream is a relatively safe topical antifungal formulation . In mutagenicity studies we found that no matter in chromosome aberration tests, or in the bone marrow micronucleus tests, Liranaftate cream has no effect on inducing cell chromosome aberrations , and it has no effect on bone marrow cell proliferation. It is used for the treatment of ringworm, athlete's foot, ringworm, jock itch, tinea versicolor and vulvovaginal candidiasis. Domestic manufacturers include: Chen Xin Shandong Lukang Pharmaceutical Co., Ltd. (Country medicine accurate words H20052287), Beijing Sihuan Pharmaceutical Technology Co., Ltd. Beijing Shengde Lai Bao Pharmaceutical Co., Ltd., Jiangsu zhongdan Pharmaceutical Co., Ltd.( Country medicine accurate words H20052733 ),Henan Tian Fang Pharmaceutical Co., Ltd. (Country medicine accurate words H20052552), and Nanjing Shenghe Pharmaceutical Co., Ltd. (Country medicine accurate words H20052554), Shideda Pharmaceutical (Beijing) Co., Ltd. (Country medicine accurate words H20052742), Kunshan Reddy pharmaceutical Co., Ltd. (Country medicine accurate words H20052345), Hugang Xinya pharmaceutical (Yangzhou) Co., Ltd. (Country medicine accurate words H20070143), Shandong Weigao pharmaceutical Co., Ltd. (Country medicine accurate words H20070073), Yangtze River pharmaceutical Group Co., Ltd. ( Country medicine accurate words H20080036), Heyuante Fukang pharmaceutical Co., Ltd. (Country medicine accurate words H20080162), Shandong Liangfu pharmaceutical Co., Ltd. (Country medicine accurate words H20080187) |
|
Uses |
Broad-spectrum antifungal |
|
Description |
Liranaftate was launched in Japan as a new topical antifungal for the treatment of dermatophycoses. This compound belonging to the thiocarbamate class of antifungals can be prepared by condensation of 5,6,7,8-tetrahydro-2-naphthol with the corresponding Npyridylthiocarbamoyl chloride. It is a potent and specific inhibitor of squalene epoxidase and consequently a blocker of ergosterol biosynthesis in fungi, without any detectable effect on mammalian cholesterol biosynthesis in rat liver at therapeutic dose levels. Liranaftate was found to be significantly more active than the other thiocarbamate tolnaftate against several dermatophytes, including Trichophyton mentagrophyfes, and against certain yeasts, such as Crypfococcus neoformans. On the other hand, it was inactive against a variety of Gram-positive and negative bacteria. When applicated as a 1 or 2% cream during clinical trials, it was well tolerated and no systemic absorption was observed. |
|
Chemical Properties |
White Crystalline Solid |
|
Originator |
Tosoh (Japan) |
|
Brand name |
Zefnart |
InChI:InChI=1/C18H20N2O2S/c1-20(16-8-5-9-17(19-16)21-2)18(23)22-15-11-10-13-6-3-4-7-14(13)12-15/h5,8-12H,3-4,6-7H2,1-2H3
88678-31-3 Relevant articles
METHOD FOR PRODUCING THIOCARBAMATE DERIVATIVE
-
, (2008/06/13)
An O-aryl N-(6-alkoxy-2-pyridyl)-N-alkyl...
METHOD FOR PRODUCING THIOCARBAMATE DERIVATIVE
-
Page/Page column 7, (2008/06/13)
An O-aryl N-(6-alkoxy-2-pyridyl)-N-alkyl...
88678-31-3 Process route
-
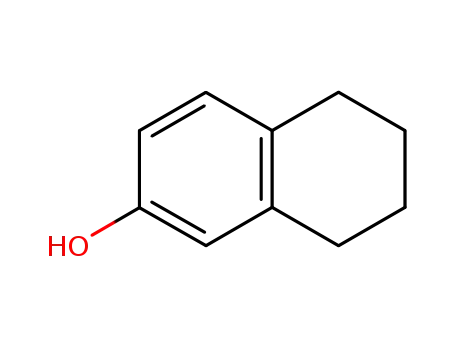
- 1125-78-6
5,6,7,8-Tetrahydro-2-naphthol

-
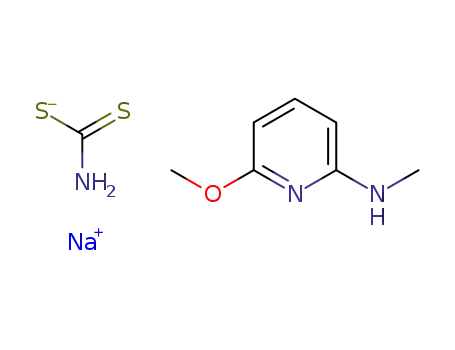
-
sodium 6-methoxy-2-methylaminopyridine dithiocarbamate

-
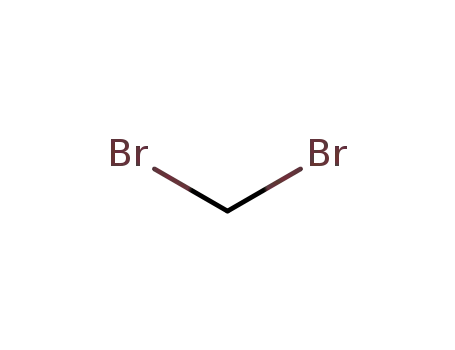
- 74-95-3
1,1-dibromomethane

-
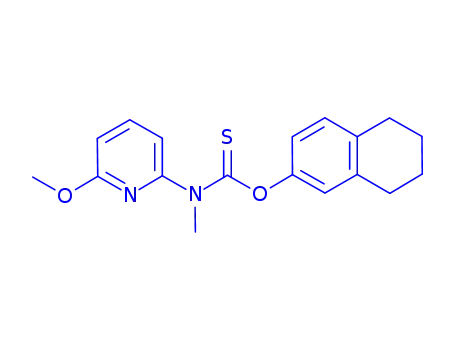
- 88678-31-3
liranaftate
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In ethyl acetate; N,N-dimethyl-formamide;
|
58% |
-
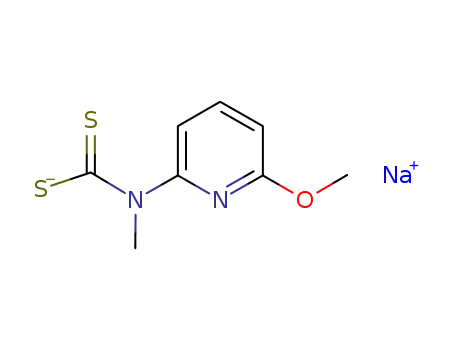
-
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt

-

- 88678-31-3
liranaftate
| Conditions | Yield |
|---|---|
|
5,6,7,8-Tetrahydro-2-naphthol; With sodium hydroxide; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt; With 1,1-dibromomethane; In N,N-dimethyl-formamide; at 10 - 20 ℃; for 1.25h; Product distribution / selectivity;
|
58% |
|
5,6,7,8-Tetrahydro-2-naphthol; With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt; With 1,1-dibromomethane; In N,N-dimethyl-formamide; at 10 - 20 ℃; for 1.25h; Product distribution / selectivity;
|
58% |
|
5,6,7,8-Tetrahydro-2-naphthol; With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt; With dichloromethane; In N,N-dimethyl-formamide; at 10 - 20 ℃; for 1.25h; Product distribution / selectivity;
|
56% |
|
5,6,7,8-Tetrahydro-2-naphthol; With sodium hydroxide; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt; With dichloromethane; In N,N-dimethyl-formamide; at 10 - 20 ℃; for 1.25h; Product distribution / selectivity;
|
55% |
|
5,6,7,8-Tetrahydro-2-naphthol; With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt; With chlorobromomethane; In N,N-dimethyl-formamide; at 10 - 20 ℃; for 1.25h; Product distribution / selectivity;
|
54% |
|
5,6,7,8-Tetrahydro-2-naphthol; With sodium hydroxide; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt; With chlorobromomethane; In N,N-dimethyl-formamide; at 10 - 20 ℃; for 1.25h; Product distribution / selectivity;
|
52% |
|
5,6,7,8-Tetrahydro-2-naphthol; With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 0.166667h;
6-methoxy-2-methylaminopyridinedithiocarbamic acid sodium salt; With diiodomethane; In N,N-dimethyl-formamide; at 10 - 20 ℃; for 1.25h; Product distribution / selectivity;
|
42% |
88678-31-3 Upstream products
-
1125-78-6

5,6,7,8-Tetrahydro-2-naphthol
-
74-95-3
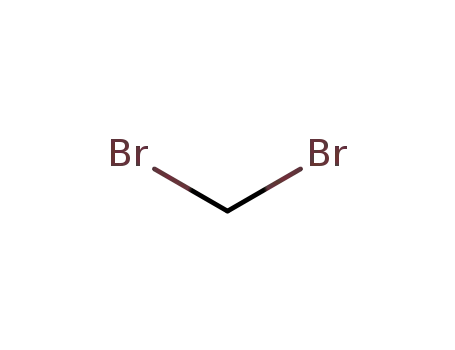
1,1-dibromomethane
Relevant Products
-
Febuxostat
CAS:144060-53-7
-
Diflorasone
CAS:2557-49-5
-
11α,17α-dihydroxy-4-prenene-3,20-dione
CAS:603-98-5