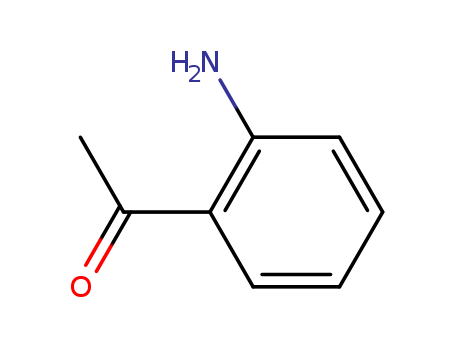
551-93-9
- Product Name:2-Aminoacetophenone
- Molecular Formula:C8H9NO
- Purity:99%
- Molecular Weight:135.166
Product Details;
CasNo: 551-93-9
Molecular Formula: C8H9NO
Appearance: yellow to yellow-brown liquid
Buy Factory Supply 2-Aminoacetophenone 551-93-9 Reasonable Price
- Molecular Formula:C8H9NO
- Molecular Weight:135.166
- Appearance/Colour:yellow to yellow-brown liquid
- Vapor Pressure:0.0258mmHg at 25°C
- Melting Point:20 °C
- Refractive Index:n20/D 1.614(lit.)
- Boiling Point:251.8 °C at 760 mmHg
- PKA:2.31±0.10(Predicted)
- Flash Point:106.1 °C
- PSA:43.09000
- Density:1.096 g/cm3
- LogP:2.05260
- IDLH:2043
2-Aminoacetophenone(Cas 551-93-9) Usage
|
Chemical Properties |
2?-Aminoacetophenone has a grape-like odor. Aminoacetophenone is a pheromone produced by virgin honeybee queens and released in feces. The pheromone repels and is used to terminate agonistic interactions between queens and workers. A grape-like odor of 2?-aminoacetophenone is of diagnostic importance in detecting the growth of Pseudomonas aeruginosa in culture and in burn wounds. |
|
Occurrence |
Reported present in chestnut honey (>154 ppb)*; green tea? and wine (0.7 to 12.8 μg/L). |
|
Uses |
2′-Aminoacetophenone may be used as an analytical standard for the determination of the analyte in livestock particulate matter, and grape-derived beverages by gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and GC-MS, respectively. |
|
Definition |
ChEBI: An aromatic ketone that is acetophenone in which one of the ortho hydrogens of the phenyl group has been replaced by an amino group. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 76, p. 4561, 1954 DOI: 10.1021/ja01647a016Journal of Heterocyclic Chemistry, 24, p. 297, 1987 DOI: 10.1002/jhet.5570240201Journal of the American Chemical Society, 105, p. 943, 1983 DOI: 10.1021/ja00342a050The Journal of Organic Chemistry, 45, p. 4926, 1980 DOI: 10.1021/jo01312a021The Journal of Organic Chemistry, 22, p. 358, 1957 DOI: 10.1021/jo01355a002 |
|
General Description |
2′-Aminoacetophenone is one of the key volatile flavor components of masa corn flour products.{9] It is also reported to be responsible for the grape-like odor in culture media growing Pseudomonas aeruginosa. |
|
Who Evaluation |
Evaluation year: 2012 |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C8H9NO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6,9H2
551-93-9 Relevant articles
Photoinduced Reduction of Nitroarenes Using a Transition-Metal-Loaded Silicon Semiconductor under Visible Light Irradiation
Tsutsumi, Ken,Uchikawa, Fumito,Sakai, Kentaro,Tabata, Kenji
, p. 4394 - 4398 (2016)
We investigated transition-metal-loaded ...
PqsBC, a condensing enzyme in the biosynthesis of the pseudomonas aeruginosa quinolone signal: Crystal structure, inhibition, and reaction mechanism
Drees, Steffen Lorenz,Li, Chan,Prasetya, Fajar,Saleem, Muhammad,Dreveny, Ingrid,Williams, Paul,Hennecke, Ulrich,Emsley, Jonas,Fetzner, Susanne
, p. 6610 - 6624 (2016)
Pseudomonas aeruginosa produces a number...
Isolation of o-acetylbenzene-amidinocarboxylic acid, a new metabolite of Gibberella saubinetii
Munekata,Seto,Tamura
, p. 1711 - 1713 (1982)
-
Synthesis of functionalised 2,3-dihydroquinolin-4(1: H)-ones vs. quinoline or N -alkenylindole derivatives through sequential reactions of 2-alkynylanilines with ketones
Marsicano, Vincenzo,Arcadi, Antonio,Chiarini, Marco,Fabrizi, Giancarlo,Goggiamani, Antonella,Iazzetti, Antonia
, p. 421 - 438 (2021)
This study describes diversity-oriented ...
Catalytic Synthesis of 3-Substituted Indoles using CO as Building Block and Supported Rhodium as Catalyst
Ucciani, Eugene,Bonfand, Andre
, p. 82 - 83 (1981)
Under hydroformylation conditions, using...
Selective hydrogenation of nitroarenes using an electrogenerated polyoxometalate redox mediator
Macdonald, Lewis,Rausch, Benjamin,Symes, Mark D.,Cronin, Leroy
, p. 1093 - 1096 (2018)
The 2-electron reduced form of the polyo...
High catalytic activity of a bimetallic AgPd alloy supported on UiO-66 derived porous carbon for transfer hydrogenation of nitroarenes using formic acid-formate as the hydrogen source
Cheng, Saisai,Shang, Ningzhao,Zhou, Xin,Feng, Cheng,Gao, Shutao,Wang, Chun,Wang, Zhi
, p. 9857 - 9865 (2017)
Bimetallic AgPd nanoparticles anchored o...
A facile reduction of aromatic nitro compounds to aromatic amines with bis(cyclopentadienyl)titanium(IV) dichloride-indium system
Yoo, Byung Woo,Lee, Sung Jae,Yoo, Byoung Seung,Choi, Kyung Il,Kim, Joong Hyup
, p. 2489 - 2493 (2002)
Cp2TiCl2/In system was found to be a new...
Palladium(II)-mediated oxidative cyclization of N-carbamoyl aminoalkynes: A new route to γ-lactams
Doan, Huynh Dong,Gore, Jacques,Vatele, Jean-Michel
, p. 6765 - 6768 (1999)
Transformation of N-carbamoyl or acetyl-...
Pd nanoparticles supported on a covalent triazine-based framework material: An efficient and highly chemoselective catalyst for the reduction of nitroarenes
Li, Jie,Zhang, Lihong,Liu, Xiaotong,Shang, Ningzhao,Gao, Shutao,Feng, Cheng,Wang, Chun,Wang, Zhi
, p. 9684 - 9689 (2018)
Pd nanoparticles anchored on a covalent ...
Intercalating ultrathin polymer interim layer for charge transfer cascade towards solar-powered selective organic transformation
Fu, Xiao-Yan,Hou, Shuo,Lin, Hua-Jian,Lin, Xin,Mo, Qiao-Ling,Wei, Zhi-Quan,Xiao, Fang-Xing,Xu, Shuai
, p. 150 - 161 (2021)
Transition metal chalcogenide quantum do...
Confinement of Quantum Dots in between Monolayered Graphene Nanosheets for Arousing Boosted Multifarious Photoredox Selective Organic Transformation
Hou, Shuo,Huang, Ming-Hui,Li, Yu-Bing,Xu, Shuai,Lin, Xin,Fu, Xiao-Yan,Xiao, Fang-Xing
, p. 16654 - 16664 (2020)
Transition metal chalcogenide quantum do...
Hollow Nano-Mesosilica Spheres Containing Rhodium Nanoparticles Supported on Nitrogen-Doped Carbon: An Efficient Catalyst for the Reduction of Nitroarenes under Mild Conditions
Wang, Shihan,Dai, Jinyu,Shi, Zhiqiang,Xiong, Zeshan,Zhang, Zongtao,Qiu, Shilun,Wang, Runwei
, p. 247 - 253 (2020)
Atom efficiency, low temperature, low pr...
Catalytic Deoxygenation of Nitroarenes Mediated by High-Valent Molybdenum(VI)-NHC Complexes
Liu, Shenyu,Amaro-Estrada, Jorge Ivan,Baltrun, Marc,Douair, Iskander,Schoch, Roland,Maron, Laurent,Hohloch, Stephan
supporting information, p. 107 - 118 (2021/02/05)
The high-valent molybdenum(VI) N-heteroc...
Cobalt nanoclusters coated with N-doped carbon for chemoselective nitroarene hydrogenation and tandem reactions in water
Agostini, Giovanni,Calvino, Jose. J.,Corma, Avelino,Gutiérrez-Tarri?o, Silvia,Lopes, Christian W.,O?a-Burgos, Pascual,Rojas-Buzo, Sergio
supporting information, p. 4490 - 4501 (2021/06/28)
The development of active and selective ...
551-93-9 Process route
-
![N-[1-(2-nitrophenyl)-1-ethoxycarbonyl]-N-(4-nitrophenyl)amine](/upload/2023/9/fbeea0bb-27b2-4253-8368-3ac37f5aa4c4.png)
-
N-[1-(2-nitrophenyl)-1-ethoxycarbonyl]-N-(4-nitrophenyl)amine

-
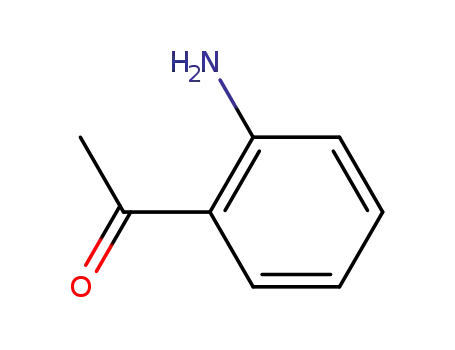
- 551-93-9
2-aminoacetophenone

-
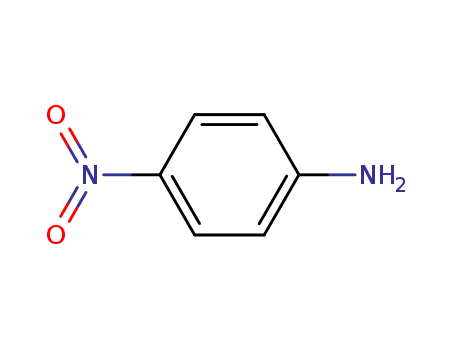
- 100-01-6,104810-17-5
4-nitro-aniline
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; for 1h; Product distribution / selectivity; Irradiation;
|
-
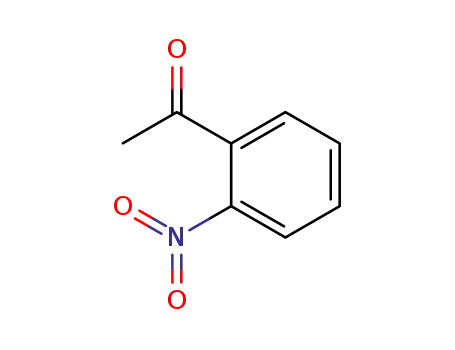
- 577-59-3
2-acetylnitrobenzene

-
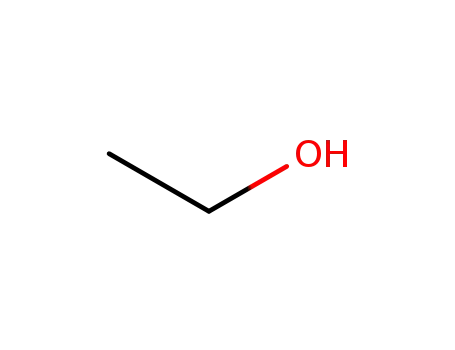
- 64-17-5
ethanol

-
- 491-35-0
C6H4NCH2CHCCH2

-
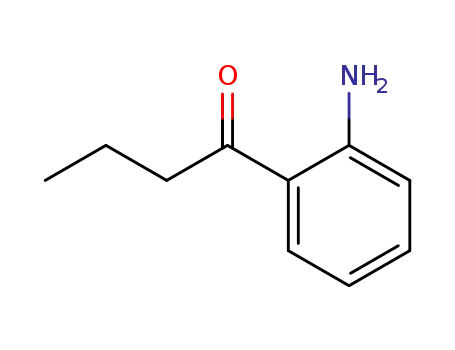
- 2034-40-4
1-(2-aminophenyl)-1-butanone

-

- 551-93-9
2-aminoacetophenone
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; at 120 ℃; for 24h;
|
6 %Chromat. 67 %Chromat. 27 %Chromat. |
551-93-9 Upstream products
-
577-59-3

2-acetylnitrobenzene
-
52670-38-9
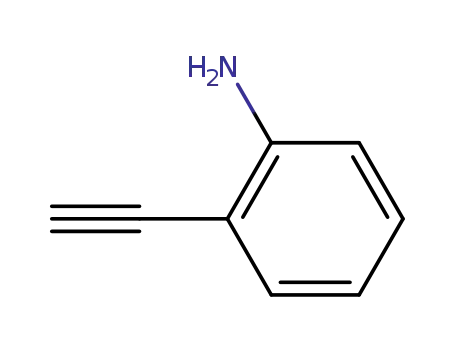
2-ethynylaniline
-
2142-68-9
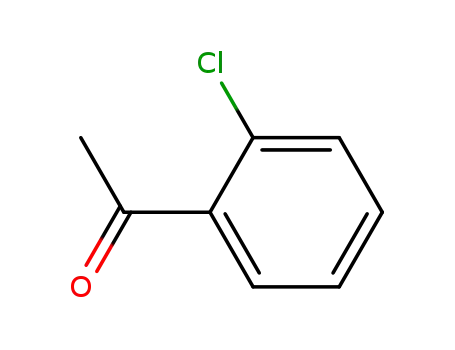
1-(2-chlorophenyl)ethanone
-
4127-53-1
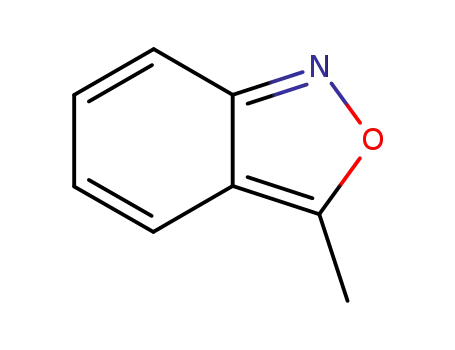
3-methylbenzo[c]isoxazole
551-93-9 Downstream products
-
874492-97-4
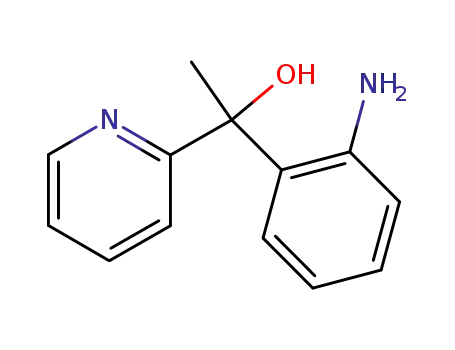
1-(2-amino-phenyl)-1-[2]pyridyl-ethanol
-
104179-53-5
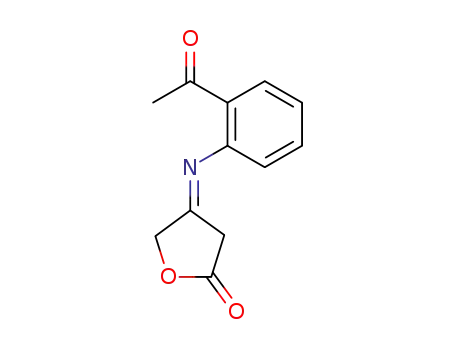
4-(2-acetyl-phenylimino)-dihydro-furan-2-one
-
143583-69-1
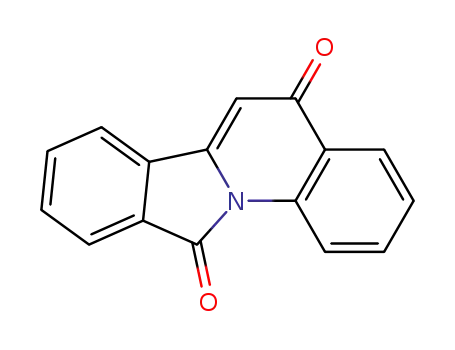
6H,12H-isoindolo[2,1-a]quinolin-6,12-dione
-
83665-31-0
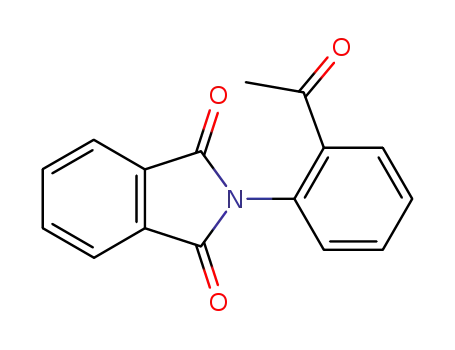
2-(2-acetylphenyl)-1H-isoindole-1,3(2H)-dione
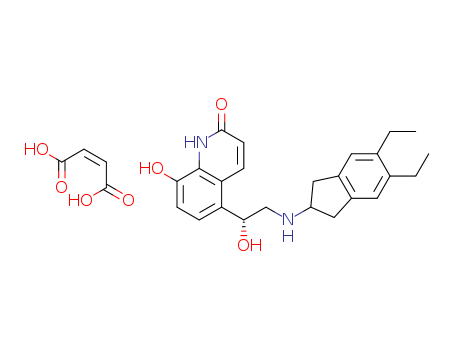
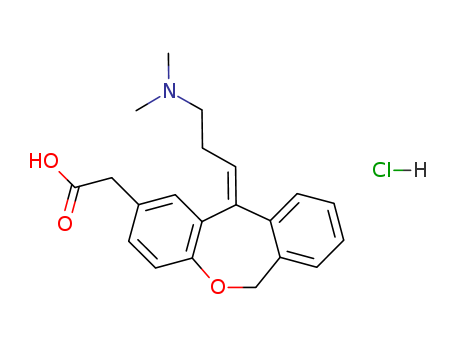
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
Indacaterol Maleate
CAS:753498-25-8
-
Olopatadine Hydrochloride
CAS:140462-76-6