140462-76-6
- Product Name:Olopatadine Hydrochloride
- Molecular Formula:C21H23NO3·HCl
- Purity:99%
- Molecular Weight:373.879
Product Details;
CasNo: 140462-76-6
Molecular Formula: C21H23NO3·HCl
Appearance: White Solid
Factory Supply High Purity Olopatadine Hydrochloride 140462-76-6 Offer with Cheap Price
- Molecular Formula:C21H23NO3·HCl
- Molecular Weight:373.879
- Appearance/Colour:White Solid
- Vapor Pressure:9.65E-13mmHg at 25°C
- Melting Point:242-245 °C
- Boiling Point:523 °C at 760 mmHg
- Flash Point:270.1 °C
- PSA:49.77000
- Density:==
- LogP:4.39150
Olopatadine hydrochloride(Cas 140462-76-6) Usage
|
Description |
Olopatadine hydrochloride is an ophthalmic antihistamine that inhibits release of histamine from mast cells and is a relatively selective histamine H1 antagonist. It inhibits type 1 immediate hypersensitivity reactions. Olopatadine hydrochloride is indicated in the temporary relief of itching caused by allergic conjunctivitis. H1 antagonists have an established and valued place in the symptomatic treatment of various immediate hypersensitivity reactions. In addition, the central properties of some of the series are of therapeutic value for suppressing motion sickness or for sedation. |
|
Chemical Properties |
White Solid |
|
Originator |
Kyowa Hakko (Japan) |
|
Uses |
Olopatadine hydrochloride is a potent, selective antagonist of the H1 histamine receptor (Ki = 16-41.1 nM), with much lower affinities for the H2 and H3 receptors (Ki = 43.4 and 172 μM, respectively). It blocks histamine-induced phosphoinositide turnover in isolated cells (IC50 = 9.5-39.9 nM) and prevents passive cutaneous anaphylaxis in rats (ED50 = 49 μg/kg) and anaphylactic bronchoconstriction in guinea pigs (ID50 = 30 μg/kg). Olopatadine is effective in treating allergic rhinitis and conjunctivitis. It also suppresses itch in patients with well-controlled chronic urticaria. In humans, this antihistamine does not cause cognitive or psychomotor impairment at therapeutic doses.[Cayman Chemical] |
|
Preparation |
Olopatadine hydrochloride can be prepared in five steps from the sodium salt of phydroxyphenylacetic acid methyl ester with phthalide. |
|
Brand name |
Patanol (Alcon). |
|
Pharmacokinetics |
Olopatadine hydrochloride was launched in the US for use in allergic conjunctivitis. It has a fast onset of action with a long duration of action (due to slow dissociation kinetics) that combines the ability to prevent human conjunctival mast cell mediator release with selective H1-receptor antangonistic activity (greater H3:H1 selectivity than H2:H1 selectivity). In addition, Olopatadine hydrochloride had no inhibition of 5-lipoxygenase, PAF acetyltransferase and thromboxane synthase while interfering with phospholipase A2. It has a presynaptic inhibition of tachykinin release and inhibits bronchial sensory nerves through activation of small conductance calcium activated potassium channels. It was more potent than ketotifen and terfenadine and was effective at inhibition of PAF, LTC4 induced conjunctivitis and TXB2 production. It does not accumulate in the CNS, has a low affinity for H1-receptors in the brain, and significantly inhibits allergen induce sneezing. Olopatadine hydrochloride was more effective in conjuctival than in corneal or the trabecuiar meshwork cells. |
|
Veterinary Drugs and Treatments |
Olopatadine HCl is a selective H1 receptor antagonist and inhibitor of histamine release from mast cells. It is marketed for topical use to alleviate symptoms of allergic conjunctivitis in humans and is thought to be safe for use in children three years of age and older. Olopatadine, upon topical application in humans, was shown to have very limited systemic absorption. It was detectable in the milk of nursing rats, after topical application, and like most medications should be avoided in pregnant or nursing animals. |
InChI:InChI=1/C21H23NO3.ClH/c1-22(2)11-5-8-18-17-7-4-3-6-16(17)14-25-20-10-9-15(12-19(18)20)13-21(23)24;/h3-4,6-10,12H,5,11,13-14H2,1-2H3,(H,23,24);1H/b18-8+;
140462-76-6 Relevant articles
Preparation method of olopatadine hydrochloride
-
Paragraph 0039; 0044-0045, (2021/11/10)
The method comprises the following steps...
A preparation method of olopatadine hydrochloride (by machine translation)
-
Paragraph 0012; 0023, (2019/10/29)
The invention discloses a method for pre...
Preparation technique of olopatadine hydrochloride
-
Paragraph 0056; 0057, (2016/10/27)
The invention relates to a preparation t...
A process for the preparation of olopatadine hydrochloride
-
, (2016/10/08)
The invention relates to a method for pr...
140462-76-6 Process route
-
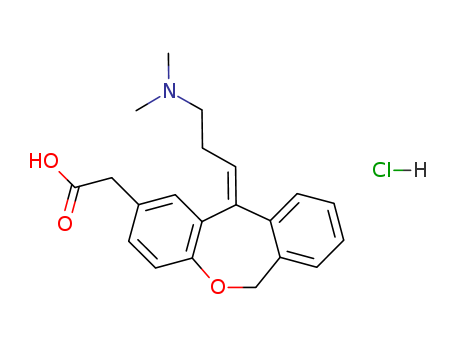
![11-hydroxy-11-(3-dimethylaminopropyl)-6,11-dihydrodibenz[b,e] oxepin-2-acetic acid](/upload/2023/9/bd678922-6385-445f-941a-d97490ca13f5.png)
-
11-hydroxy-11-(3-dimethylaminopropyl)-6,11-dihydrodibenz[b,e] oxepin-2-acetic acid

-
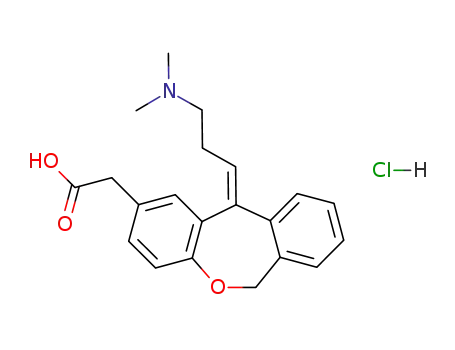
-
140462-76-6
olopatadine hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; acetic anhydride;
In
toluene;
at 100 ℃;
for 6h;
Sealed tube;
|
79.5% |
-
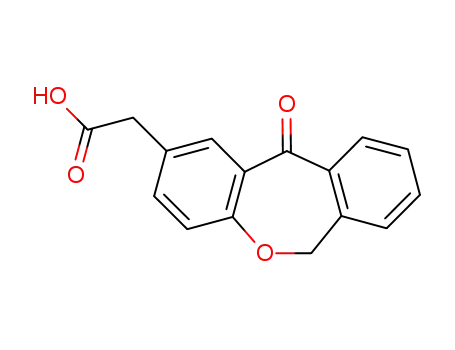
-
55453-87-7
isoxepac

-
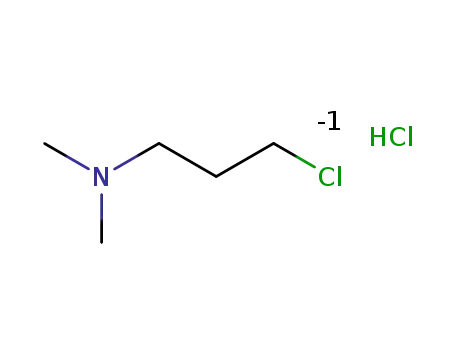
-
N-(3-chloropropyl)-N,N-dimethylamine hydrochloride

-

-
140462-76-6
olopatadine hydrochloride
| Conditions | Yield |
|---|---|
|
N-(3-chloropropyl)-N,N-dimethylamine hydrochloride;
With
potassium bromide;
for 1h;
Inert atmosphere;
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
In
dimethyl sulfoxide;
for 2h;
Reflux;
isoxepac;
In
dimethyl sulfoxide;
at 20 ℃;
for 1h;
Reagent/catalyst;
Solvent;
|
90.5% |
140462-76-6 Upstream products
-
113806-05-6
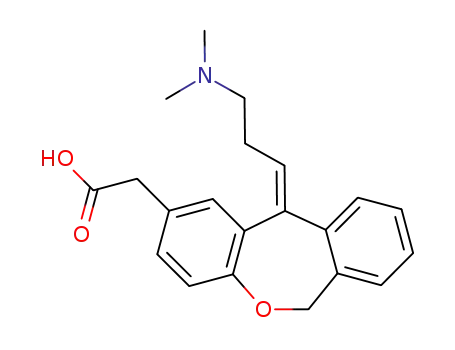
olopatadine
-
60548-16-5
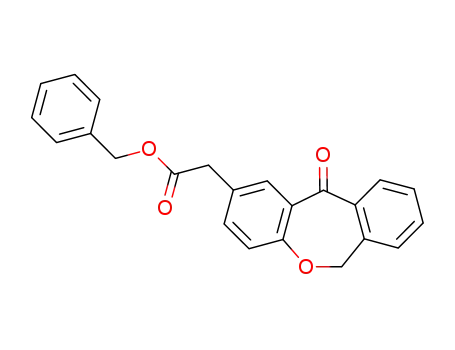
(11-oxo-6,11-dihydro-dibenzo[b,e]oxepin-2-yl)-acetic acid benzyl ester
-
27710-82-3
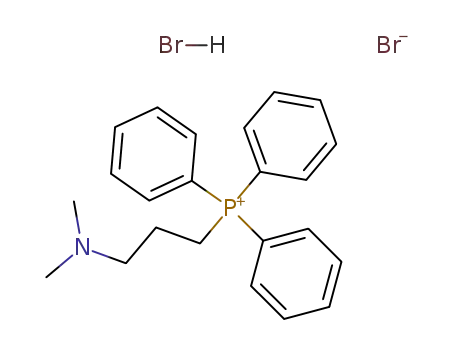
anhydrous 3-(dimethylamino)propyltriphenylphosphonium bromide hydrobromide
-
113806-03-4
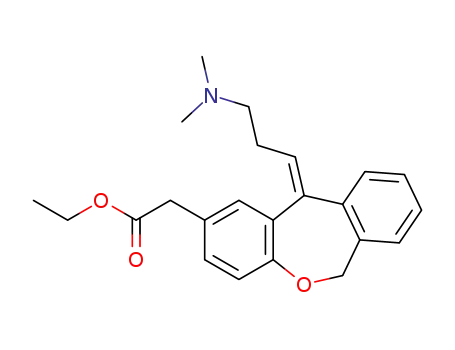
(Z)-11-(3-dimethylaminopropylidene)-6,11-dihydrodibenz[b,e] oxepin-2-acetic acid ethyl ester
Relevant Products
-
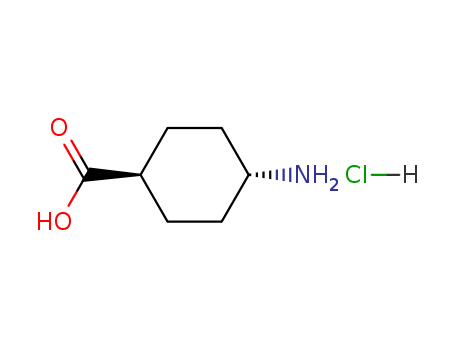
Trans-4-Amino-Cyclohexane Carboxylic Acid Hydrochloride
CAS:27960-59-4
-
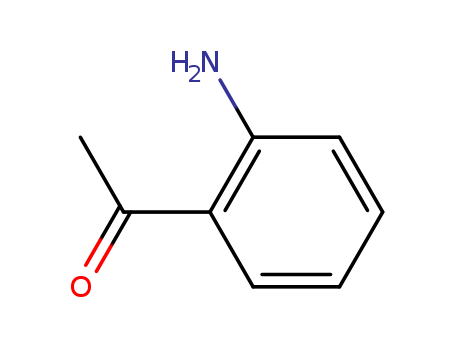
2-Aminoacetophenone
CAS:551-93-9
-
Nilotinib HCl
CAS:923288-90-8