141625-93-6
- Product Name:Dronedarone Hydrochloride
- Molecular Formula:C31H44N2O5S.HCl
- Purity:99%
- Molecular Weight:593.228
Product Details;
CasNo: 141625-93-6
Molecular Formula: C31H44N2O5S.HCl
Quality Factory Customized Supply 99% Pure Dronedarone Hydrochloride 141625-93-6
- Molecular Formula:C31H44N2O5S*ClH
- Molecular Weight:593.228
- Vapor Pressure:1.47E-18mmHg at 25°C
- Melting Point:NA (low-melting)
- Boiling Point:683.9 °C at 760 mmHg
- Flash Point:367.4 °C
- PSA:97.23000
- LogP:9.00480
DRONEDARONE HYDROCHLORIDE(Cas 141625-93-6) Usage
|
Chemical Properties |
Pale Yellow Solid |
|
Uses |
Dronedarone Hydrochloride is a therapy for the treatment of patients with paroxysmal and persistent atrial fibrillation or atrial flutter. |
|
Biological Activity |
dronedarone hcl is an amiodarone analogue which has been shown an effective and promising treatment for atrial fibrillation (af) [1]. |
|
Biochem/physiol Actions |
Dronedarone is a Class III antiarrhythmic and a multi-channel blocker for atrial fibrillation. It blocks potassium, sodium, and calcium channels and also exhibits antiadrenergic properties. |
|
Clinical Use |
Dronedarone hydrochloride (also known as SR33589 and marketed as Multaq) is a drug developed by Sanofi-Aventis for cardiac arrhythmias (irregular heartbeat) that was approved by the FDA in July 2009. Dronedarone is used for the treatment of atrial fibrillation and atrial flutter in patients whose hearts have either returned to normal rhythm or who undergo drug therapy or electroshock treatment to maintain normal cardio rhythm. Dronedarone is less lipophilic than amiodarone, exhibits a much smaller volume of distribution and a half-life of 24 h, this stands in contrast to competitor amiodarone’s half-life of several weeks. As a result of these pharmacokinetic characteristics, dronedarone dosing may be less complicated than amiodarone. |
|
Synthesis |
The synthesis of dronedarone relies on the preparation of the benzofuran core 34, of which three main routes have been reported, but two possess obvious overlap and are considered more process-amenable. Starting from methyl 2-(2-formylphenoxy)hexanoate (32), this aldehyde can either be nitrated, then saponified or saponified and then nitrated to procure nitroacid 33 (the Scheme). The benzofuran ring is then secured through the use of acetic anhydride and base in the presence of DMF at elevated temperature. The key benzofuran 34 can be produced by either route in 62% yield on gramscale by this method. Friedel–Crafts acylation involving anisoyl chloride and tin tetrachloride constructed the diaryl ketone 35. Cleavage of the methyl ether through the use of aluminum trichloride in refluxing DCE provided phenol 36. Alkylation of phenol 36 with aminoalkyl chloride 37 gave ether 38. Subsequent reduction of the nitro group via catalytic hydrogenation and sulfonylation of the resulting amine provided dronedarone (VII) which was isolated as its HCl salt. |
|
in vitro |
dronedarone has been demonstrated to inhibit muscarinic acetylcholine receptor-operated k+ current ik(ach) induced by carbachol or gtp-gamma-s with ic50 values of 10nm and <100nm, respectively, in cells isolated from guinea pig atria. notably, dronedarone was 100-fold potent and selective over amiodarone in inhibiting ik(ach) [1]. |
|
in vivo |
dronedarone has shown to block arterial thrombus formation, decrease platelet aggregation and reduce plasminogen activator inhibitor-1 (pai1) expression in c57bl/6 mice [2]. |
|
references |
[1] guillemare e1, marion a, nisato d, gautier p. inhibitory effects of dronedarone on muscarinic k+ current in guinea pig atrial cells. j cardiovasc pharmacol. 2000 dec;36(6):802-5.[2] breitenstein a1, sluka sh, akhmedov a, stivala s, steffel j, camici gg, riem hh, beer hj, studt jd, duru f, luscher tf, tanner fc. dronedarone reduces arterial thrombus formation. basic res cardiol. 2012 nov;107(6):302. |
InChI:InChI=1/C31H44N2O5S.ClH/c1-5-8-12-29-30(27-23-25(32-39(4,35)36)15-18-28(27)38-29)31(34)24-13-16-26(17-14-24)37-22-11-21-33(19-9-6-2)20-10-7-3;/h13-18,23,32H,5-12,19-22H2,1-4H3;1H
141625-93-6 Relevant articles
An improved scalable route to pure dronedarone hydrochloride
Hivarekar, Raghvendra R.,Deshmukh, Sanjay S.,Tripathy., Narendra K.
, p. 677 - 681 (2012)
An efficient scalable synthesis for dron...
An improved and efficient process for the production of dronedarone hydrochloride, an antiarrhythmia drug
Mali, Anil C.,Ippar, Sharad S.,Bodke, Mahendra B.,Patil, Nilesh S.,Mathad, Vijayavitthal T.
, p. 863 - 868 (2013)
An improved, high-yielding, and efficien...
Preparation method for dronedarone hydrochloride
-
Paragraph 0070-0072; 0077-0085, (2019/03/15)
The invention relates to a preparation m...
A short synthesis of Dronedarone
Piotrkowska, Barbara,Nerdinger, Sven,Schreiner, Erwin,Seli?, Lovro,Graczyk, Piotr P.
, p. 4330 - 4335 (2018/05/04)
A modification of the Nenitzescu reactio...
Method for synthesizing [...] reach silom
-
Paragraph 0092-0097, (2017/01/17)
The invention discloses a dronedarone sy...
METHOD FOR PREPARING 5-AMINO-BENZOFURAN DERIVATIVES
-
Paragraph 0041, (2015/02/19)
The invention relates to a method for pr...
141625-93-6 Process route
-
![4-[3-(di-n-butylamino)-propoxy]-benzoic acid chloride hydrochloride](/upload/2023/9/67aded45-2af5-476e-87eb-9435b0fa07e0.png)
-
4-[3-(di-n-butylamino)-propoxy]-benzoic acid chloride hydrochloride

-
-
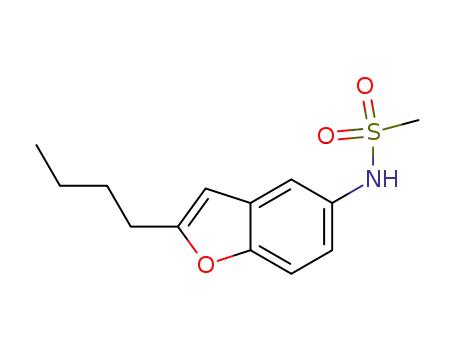
437652-07-8
N-(2-butyl-2,3-dihydro-5-benzofuranyl)methanesulfonamide

-
-
141625-93-6
dronedarone hydrochloride
| Conditions | Yield |
|---|---|
|
With
iron(III) chloride;
In
dichloromethane;
for 2h;
Heating / reflux;
|
90% |
|
aluminum (III) chloride;
In
dichloromethane;
at 25 ℃;
for 2h;
|
90% |
|
4-[3-(di-n-butylamino)-propoxy]-benzoic acid chloride hydrochloride; N-(2-butyl-2,3-dihydro-5-benzofuranyl)methanesulfonamide;
With
aluminum (III) chloride; tin(IV) chloride;
In
dichloromethane;
at 20 ℃;
for 3h;
With
hydrogenchloride;
|
-
![3-{4-[(2-butyl-5-methanesulfonamide-1-benzofuran-3-yl)carbonyl]phenoxy}propyl methanesulfonate](/upload/2023/9/39380458-882e-4e0f-9599-6be9f7261cd4.png)
-
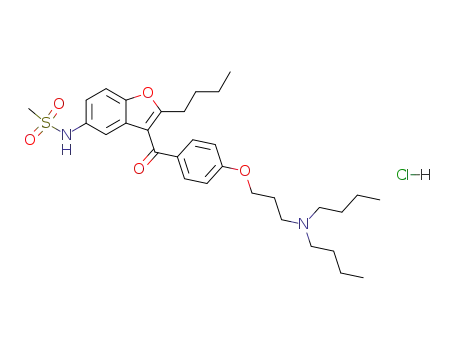
1310430-09-1
3-{4-[(2-butyl-5-methanesulfonamide-1-benzofuran-3-yl)carbonyl]phenoxy}propyl methanesulfonate

-
-
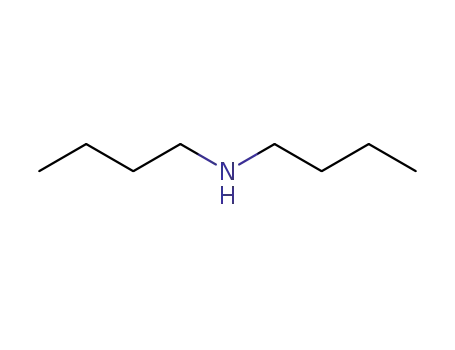
111-92-2
dibutylamine

-

-
141625-93-6
dronedarone hydrochloride
| Conditions | Yield |
|---|---|
|
3-{4-[(2-butyl-5-methanesulfonamide-1-benzofuran-3-yl)carbonyl]phenoxy}propyl methanesulfonate; dibutylamine;
In
acetonitrile;
Reflux;
With
hydrogenchloride;
In
water; acetone;
at 40 ℃;
for 2h;
|
71% |
|
3-{4-[(2-butyl-5-methanesulfonamide-1-benzofuran-3-yl)carbonyl]phenoxy}propyl methanesulfonate; dibutylamine;
In
acetonitrile;
Reflux;
With
hydrogenchloride;
In
water; acetone;
at 0 - 40 ℃;
|
71% |
|
Multi-step reaction with 2 steps
1: butanone / 16 h
2: hydrogenchloride / isopropyl alcohol; water / 5 h / 0 °C
With
hydrogenchloride;
In
water; isopropyl alcohol; butanone;
|
141625-93-6 Upstream products
-
437652-07-8

N-(2-butyl-2,3-dihydro-5-benzofuranyl)methanesulfonamide
-
141626-36-0
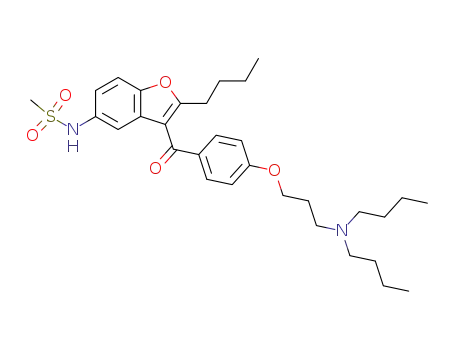
dronedarone
-
1310430-09-1
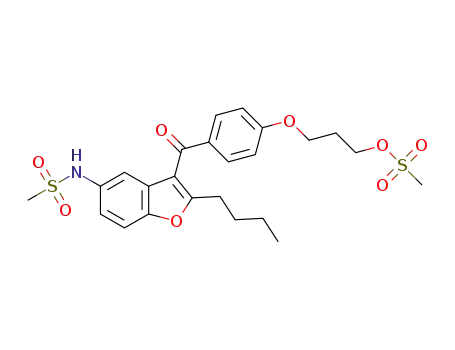
3-{4-[(2-butyl-5-methanesulfonamide-1-benzofuran-3-yl)carbonyl]phenoxy}propyl methanesulfonate
-
111-92-2

dibutylamine
141625-93-6 Downstream products
-
1354567-97-7
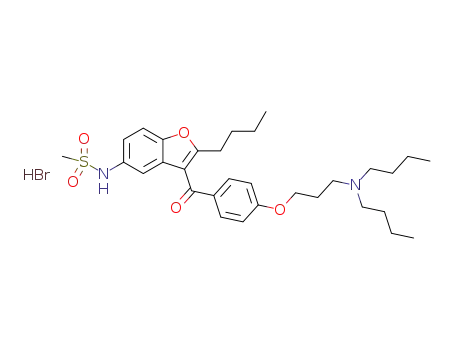
N-[2-butyl-3-[4-(3-(di-n-butylamino)propoxy)benzoyl]-5-benzofuranyl]methanesulfonamide hydrobromide
Relevant Products
-
Bepotastine Besilate
CAS:190786-44-8
-
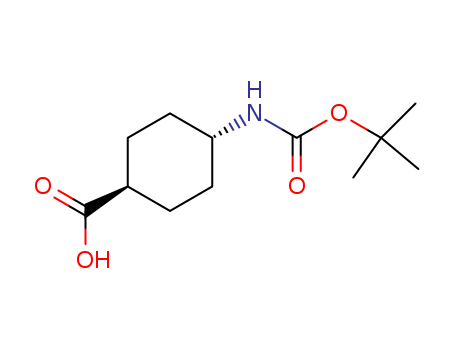
Trans-4-(boc-amino)cyclohexanecarboxylic acid
CAS:53292-89-0
-
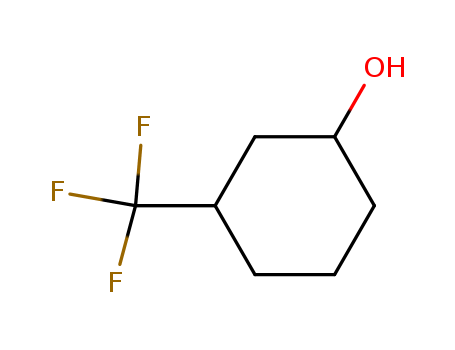
3-(Trifluoromethyl)Cyclohexanol
CAS:454-63-7