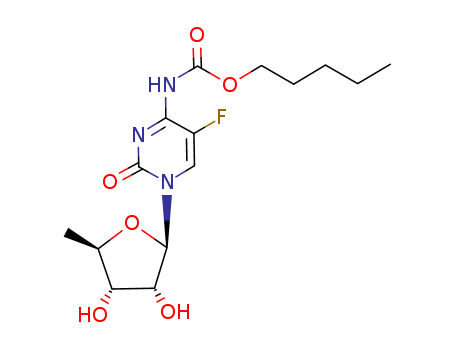
154361-50-9
- Product Name:Capecitabine
- Molecular Formula:C15H22FN3O6
- Purity:99%
- Molecular Weight:359.355
Product Details;
CasNo: 154361-50-9
Molecular Formula: C15H22FN3O6
Appearance: Colourless solid
Buy High Quality Reliable Quality Capecitabine 154361-50-9 Reasonable Price
- Molecular Formula:C15H22FN3O6
- Molecular Weight:359.355
- Appearance/Colour:Colourless solid
- Vapor Pressure:0mmHg at 25°C
- Melting Point:110-121 °C
- Refractive Index:1.7
- Boiling Point:437.874oC at 760 mmHg
- PKA:5.41±0.40(Predicted)
- Flash Point:218.619oC
- PSA:122.91000
- Density:1.49 g/cm3
- LogP:0.83320
Capecitabine(Cas 154361-50-9) Usage
|
Clinical trials |
Analyses of nearly 400 randomized comparisons of clinical outcomes show that a combination of adriamycin, cisplatin or oxaliplatin with 5-FU, compared to a combination of these drugs with Capecitabine, has a two-fold difference in curative efficacy and lowers toxicty. A large-scale Chinese clinical trial of Capecitabine in combination with DDP in the treatment of stage II gastric cancer also proves its advantages of high efficacy, low toxicity, and affordability. |
|
Drug interactions |
Currently, there are no side effects of clinical significance when used in combination with antihistamines, NSAIDs, morphine, paracetamol, aspirin, antiemetic drugs, and H2 receptor antagonist drugs. Capecitabine’s binding rate with serum protein is relatively low (64%), and its possibility of interacting through substitution with drugs that bind closely with proteins is currently unknown. In external experiments, Capecitabine has not shown any influence on human liver microsomal P450 enzyme. If any phenytoin and coumarin derivatives anticoagulants are used in combination with Capecitabine, dosages should be lowered. |
|
Adverse effects |
Common adverse reactions include nausea, vomiting, oral ulcers, abdominal pain, diarrhea, loss of appetite, and skin changes. There have also been reports of some patients experiencing transient myelosuppression, hair loss, tears, headache, and dizziness. |
|
Warnings and precautions |
Capecitabine is a bone marrow inhibitor; a blood exam must be administered before every usage to monitor blood cell and platelet count. This product poses toxic side effects to the liver, so liver functions must be routinely examined. Additionally, heart functions should also be monitored to prevent irreversible toxic reactions. If venous transfusion of the drug is required, the aforementioned organ functions should be tested for suitability before administration. Capecitabine may cause damage to embryos, thus making it unsuitable for pregnant women. Women using this drug are also not suitable for pregnancy. Even after treatment is ended, this drug may have some impact on fertility. |
|
Description |
Capecitabine is an oral fluorinated pyrimidine drug developed by Roche Pharmaceuticals and marketed under the commercial name Xeloda. It is used as an antineoplastic agent, primarily for the treatment of various forms of cancer. |
|
Chemical Properties |
Colourless solid |
|
Originator |
Roche (Switzerland) |
|
Uses |
Capecitabine is an oral chemotherapy drug that is converted into 5-FU in the body, specifically at tumor sites, making it a valuable treatment option for various forms of cancer, including breast, colorectal, and other solid tumors. |
|
Definition |
ChEBI: A carbamate ester that is cytidine in which the hydrogen at position 5 is replaced by fluorine and in which the amino group attached to position 4 is converted to its N-(penyloxy)carbonyl derivative. Capecitabine is a antineoplastic agen used in the treatment of cancers. |
|
Manufacturing Process |
5-Deoxy-5-fluoro-N4-((n-pentyloxy)carbonyl)cytidine may be prepared according to US Patent No. 6,114,520.From 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-fluorocytidine and n-pentylchloroformate in dichloromethane and pyridine may be obtained 2',3'- bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-fluoro-N4-((pentyloxy)carbonyl) cytidine.From 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-fluoro-N4- ((pentyloxy)carbonyl)cytidineand tetrabutylammonium fluoride in tetrahydrofuran at room temperature for 2 hours may be prepared the product which by hydrolyses may be converted to 5-deoxy-5-fluoro-N4- ((pentyloxy)carbonyl)cytidine. Purification of the product may be carried out by silica gel chromatography (using dichloromethane:methanol = 20:1 as an eluent). |
|
Brand name |
Xeloda (Roche). |
|
Therapeutic Function |
Antitumor |
|
Biochem/physiol Actions |
Capecitabine is an anti-cancer drug, a prodrug of doxifluridine, metabolized to 5-fluorouracil at the tumor site. The activation of capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5′-Deoxy-5-fluorocytidine (5′-DFCR) and 5′-Deoxy-5-fluorouridine (5′-DFUR), to form 5-fluorouracil. |
|
Clinical Use |
Capecitabine is indicated for use as first-line therapy in patients with colorectal cancer. It also is used alone or in combination with docetaxel in patients with metastatic breast cancer who have experienced disease progression or recurrence after anthracycline therapy. Given b.i.d. in tablet form, the total daily dose is calculated based on patient body surface area and is taken 30 minutes after eating to avoid food-induced decreases in absorption. In addition to bone marrow suppression, nausea, and vomiting, the drug can induce severe diarrhea and a potentially disabling disorder termed “hand-and-foot syndrome” (palmar-plantar erythrodysethesia). Capecitabine inhibits CYP2C9 and, along with competition for serum protein binding sites, results in clinically significant drug–drug interactions with both warfarin and phenytoin. |
|
in vitro |
in antiproliferative assays, both ls174t wt and ls174t-c2 cells were more sensitive to capecitabine when cultivated in the same plates as hepg2 hepatoma. in ls174t wt alone and cultivated with hepg2, ic50values of capecitabine were 890 ± 48 and 630 ± 14 μm respectively. the ic50fell from 330 ± 4 down to 89 ± 6 μmin ls174t-c2 subline when cultivated in the same plates as hepatoma cells.in the ls174t-c2 subclone, whereas little cell death occurred in cells exposed to capecitabine, both early and late apoptosis were increased by 244 and 262%, respectively [1]. furthermore, capecitabine induces apoptosis in a fas-dependent manner, and shows a 7-fold higher cytotoxicity and markedly stronger apoptotic potential in thymidine phosphorylase (tp)-transfected ls174t-c2 cells [2]. |
|
in vivo |
capecitabine was effective in a wider dose range in cxf280, hct116, colo205, and widr human colon cancer xenograft models [2]. in highly metastatic nude mice model, capecitabine inhibited tumor growth and metastatic recurrence after resection of hcc attributed to the high expression of pd-ecgf in tumors [3]. |
InChI:InChI=1/C9H12FN3O4/c1-3-5(14)6(15)8(17-3)13-2-4(10)7(11)12-9(13)16/h2-3,5-6,8,14-15H,1H3,(H2,11,12,16)/t3-,5-,6-,8-/m1/s1
154361-50-9 Relevant articles
Synthesis of the antitumoral nucleoside capecitabine through a chemo-enzymatic approach
Ciceri, Samuele,Ciuffreda, Pierangela,Grisenti, Paride,Ferraboschi, Patrizia
, p. 5909 - 5913 (2015)
Capecitabine is a 5′-deoxynucleoside end...
Continuous-Flow Sequential Schotten-Baumann Carbamoylation and Acetate Hydrolysis in the Synthesis of Capecitabine
Miranda, Leandro S. De M.,De Souza, Rodrigo O. M. A.,Lea?, Raquel A. C.,Carneiro, Paula F.,Pedraza, Sergio F.,De Carvalho, Otavio V.,De Souza, Stefania P.,Neves, Rebeca V.
, p. 2516 - 2520 (2019)
Capecitabine is an important anticancer ...
Cytidine derivative and method for preparing capecitabine medicines through derivative
-
Paragraph 0065-0069, (2020/05/14)
The invention discloses a 5-deoxy-D-ribo...
154361-50-9 Process route
-
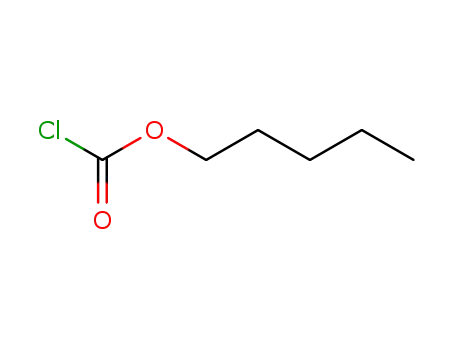
- 638-41-5
pentyl chloroformate

-
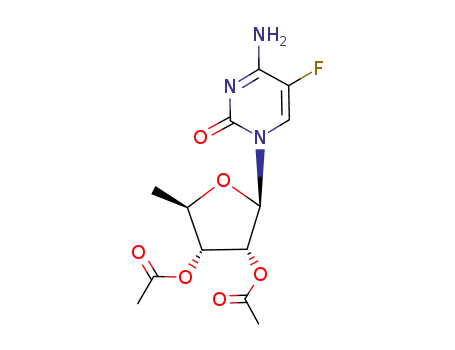
- 161599-46-8,2230479-30-6
(2R,3R,4R,5R)-2-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-5-methyl-tetrahydrofuran-3,4-diyl diacetate

-
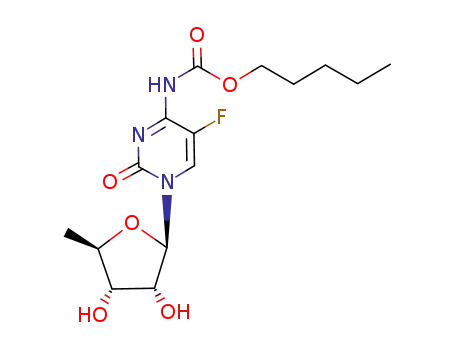
- 154361-50-9,158798-73-3
capecitabine
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; acetone; at 30 ℃; for 5h; Green chemistry;
|
82% |
|
pentyl chloroformate; (2R,3R,4R,5R)-2-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-5-methyl-tetrahydrofuran-3,4-diyl diacetate; With pyridine; In dichloromethane; at 0 ℃; for 1h;
With sodium hydroxide; In ethanol; water; at -5 - 0 ℃; for 0.233333h;
|
80.2% |
|
Multi-step reaction with 2 steps
1: dichloromethane
2: sodium hydroxide / methanol
With sodium hydroxide; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: pyridine / dichloromethane / 1 h / 0 °C
2: sodium methylate / methanol / 1 h / 20 °C
With pyridine; sodium methylate; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: potassium phosphate / isopropyl alcohol; dichloromethane / 4 h / 0 - 25 °C / Inert atmosphere
2: sodium hydroxide / methanol; water / 1 h / -10 - 5 °C / Autoclave
With potassium phosphate; sodium hydroxide; In methanol; dichloromethane; water; isopropyl alcohol;
|
-
-
2'3'-O-isopropylidene-5'-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine

-

- 154361-50-9,158798-73-3
capecitabine
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid; In methanol; dichloromethane; water; at 10 - 20 ℃; for 3h; Solvent;
|
89.4% |
154361-50-9 Upstream products
-
162204-20-8
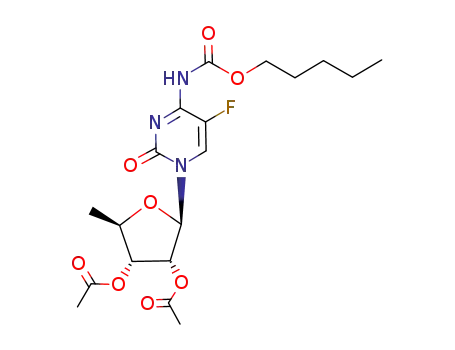
N1-(2',3'-di-O-acetyl-5'-deoxy-β-D-ribofuranosyl)-5-fluoro-N4-(pentyloxycarbonyl)cytosine
-
638-41-5

pentyl chloroformate
-
161599-46-8

(2R,3R,4R,5R)-2-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-5-methyl-tetrahydrofuran-3,4-diyl diacetate
-
27821-07-4
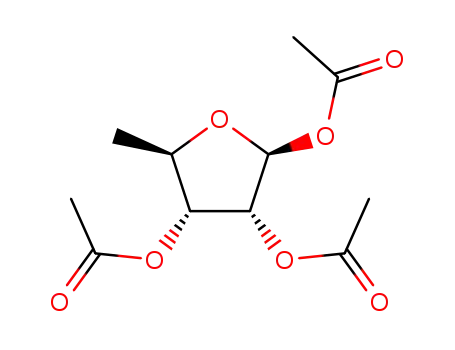
1,2,3-tri-O-acetyl-5-deoxy-β-D-ribofuranose
154361-50-9 Downstream products
-
66335-38-4
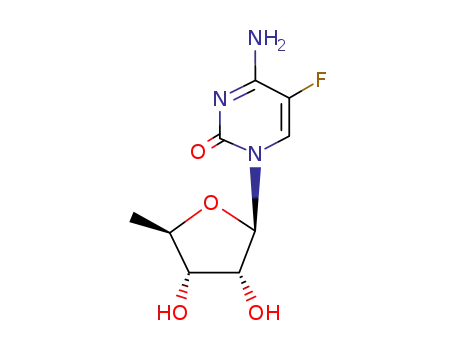
5'-deoxy-5-fluorocytidine
Relevant Products
-
Voriconazole
CAS:137234-62-9
-
Ursodeoxycholic Acid
CAS:128-13-2
-
3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile
CAS:35202-54-1