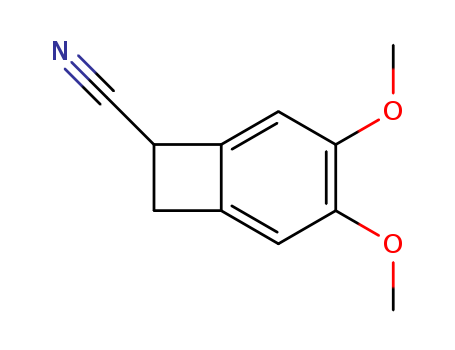
35202-54-1
- Product Name:3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile
- Molecular Formula:C11H11NO2
- Purity:99%
- Molecular Weight:189.214
Product Details;
CasNo: 35202-54-1
Molecular Formula: C11H11NO2
Buy High Quality Top Purity 3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile 35202-54-1 In Bulk Supply
- Molecular Formula:C11H11NO2
- Molecular Weight:189.214
- Vapor Pressure:5.98E-05mmHg at 25°C
- Melting Point:83-84 °C
- Refractive Index:1.557
- Boiling Point:345.9 °C at 760 mmHg
- Flash Point:140.5 °C
- PSA:42.25000
- Density:1.18 g/cm3
- LogP:1.86708
4,5-Dimethoxy-1-cyanobenzocyclobutane(Cas 35202-54-1) Usage
| Description | 4,5-Dimethoxy-1-cyanobenzocyclobutane is an intermediate in the synthesis of ivabradine. It is produced by reacting 4,5-dimethoxybenzoic acid with cyanoacetic acid in the presence of acetonitrile and potassium carbonate in a refluxing solution. |
|
Uses |
4,5-Dimethoxy-1-cyanobenzocyclobutane is used in the preparation of selective bradycardic agents. |
InChI:InChI=1/C11H11NO2/c1-13-10-4-7-3-8(6-12)9(7)5-11(10)14-2/h4-5,8H,3H2,1-2H3
35202-54-1 Relevant articles
Process for the enzymatic synthesis of (7S)-3,4-dimethoxybicyclo[4.2.0]OCTA-1,3,5-triene-7-carboxylic acid and application in the synthesis of ivabradine and salts thereof
-
Page/Page column 8, (2016/11/14)
Process for the enzymatic synthesis of t...
PROCESS FOR THE SYNTHESIS OF (2E)-3-(3,4-DIMETHOXYPHENYL)PROP-2-ENENITRILE, AND APPLICATION IN THE SYNTHESIS OF IVABRADINE AND ADDITION SALTS THEREOF WITH A PHARMACEUTICALLY ACCEPTABLE ACID
-
Paragraph 0040-0043, (2014/05/20)
Process for the synthesis of the compoun...
35202-54-1 Process route
-
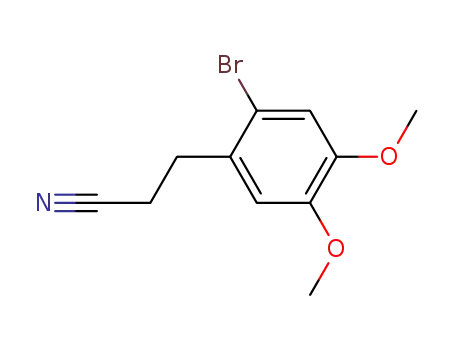
- 35249-62-8
3-(2-bromo-4,5-dimethoxyphenyl)propanenitrile

-
![3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile](/upload/2023/9/c3e4a87a-4d1b-4730-9fc2-c61523f72481.png)
- 35202-54-1
3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile
| Conditions | Yield |
|---|---|
|
With n-butyllithium; In tetrahydrofuran; hexane; at -10 ℃; Inert atmosphere;
|
80% |
|
With ammonia; sodium amide; at -45 ℃; for 2h;
|
74% |
|
With sodium amide; In ammonia; at 20 ℃; for 2h; liquid NH3;
|
74% |
|
With iron(III) chloride; ammonia; sodium; at 20 ℃; for 2h;
|
74% |
|
With sodium amide; at 20 ℃; for 2h;
|
74% |
-
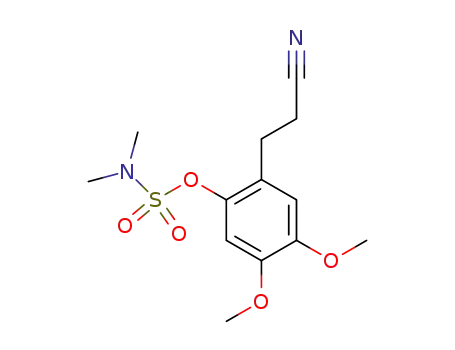
- 1232191-48-8
2-(2-cyanoethyl)-4,5-dimethoxyphenyl dimethylsulphamate

-
![3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile](/upload/2023/9/c3e4a87a-4d1b-4730-9fc2-c61523f72481.png)
- 35202-54-1
3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile
| Conditions | Yield |
|---|---|
|
2-(2-cyanoethyl)-4,5-dimethoxyphenyl dimethylsulphamate; With n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at -60 - -5 ℃; Inert atmosphere;
With water; In tetrahydrofuran; hexane;
|
50% |
35202-54-1 Upstream products
-
84452-13-1
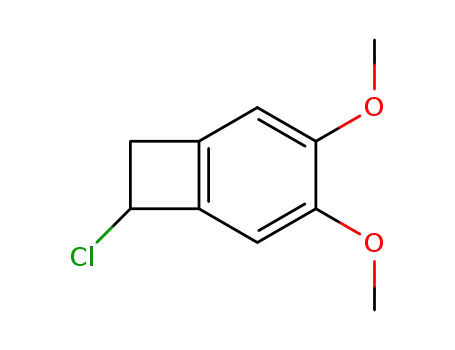
7-Chloro-3,4-dimethoxy-bicyclo[4.2.0]octa-1(6),2,4-triene
-
7721-62-2
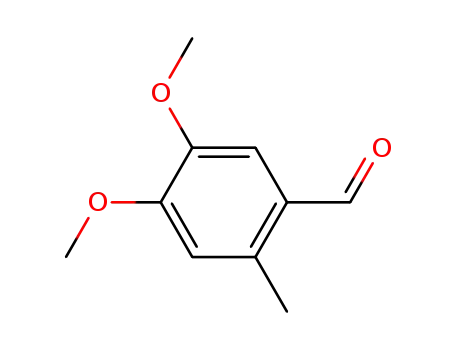
4,5-dimethoxy-2-methylbenzaldehyde
-
84452-08-4
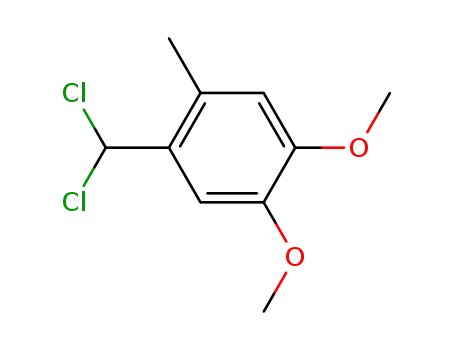
1-Dichloromethyl-4,5-dimethoxy-2-methyl-benzene
-
35249-62-8

3-(2-bromo-4,5-dimethoxyphenyl)propanenitrile
35202-54-1 Downstream products
-
54089-46-2
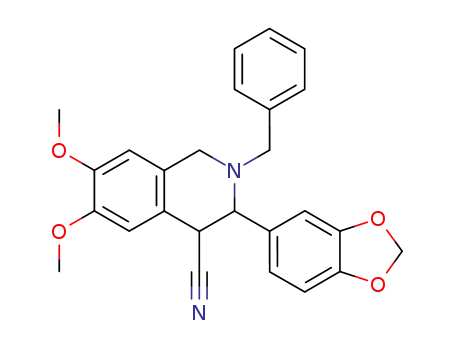
3-benzo[1,3]dioxol-5-yl-2-benzyl-6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-4-carbonitrile
-
73344-75-9
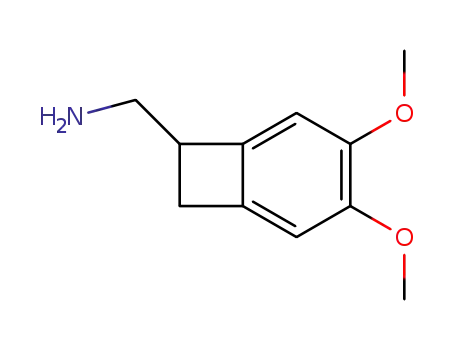
(±)-(3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methylamine
-
866783-13-3
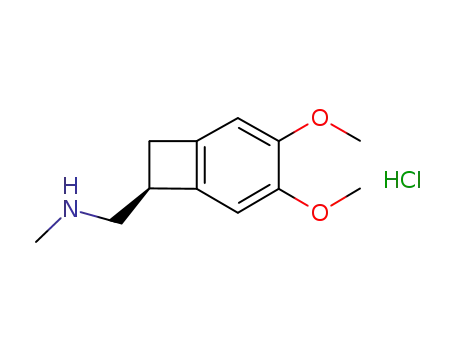
1-[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]-N-methylmethanamine hydrochloride
-
148849-67-6
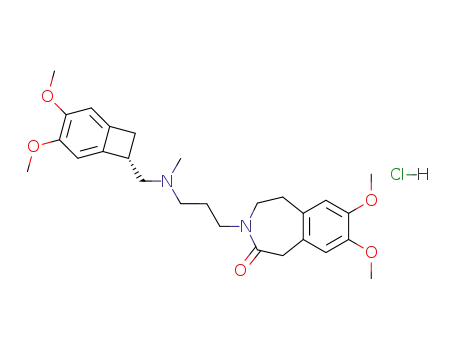
ivabradine hydrochloride
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
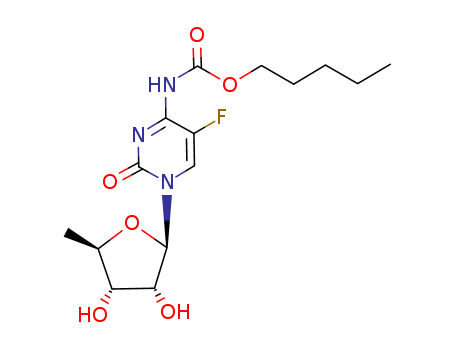
Capecitabine
CAS:154361-50-9
-
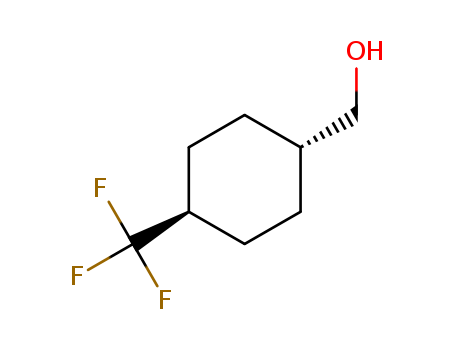
trans-(4-(trifluoromethyl)cyclohexyl)methanol
CAS:1202577-61-4