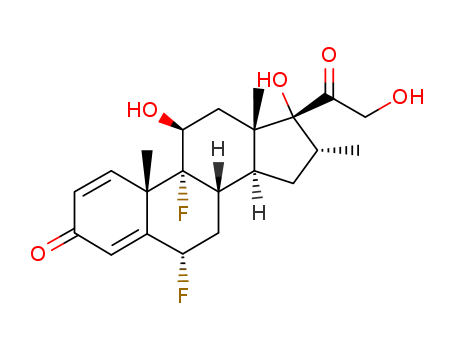
2135-17-3
- Product Name:Flumethasone
- Molecular Formula:C22H28F2O5
- Purity:99%
- Molecular Weight:410.458
Product Details;
CasNo: 2135-17-3
Molecular Formula: C22H28F2O5
Appearance: White crystalline powder
Quality Manufacturer Supply Wholesale Flumethasone 2135-17-3 Fast Shipping
- Molecular Formula:C22H28F2O5
- Molecular Weight:410.458
- Appearance/Colour:White crystalline powder
- Vapor Pressure:2.33E-15mmHg at 25°C
- Melting Point:237-240oC
- Refractive Index:1.579
- Boiling Point:569.8 °C at 760 mmHg
- PKA:11.98±0.70(Predicted)
- Flash Point:298.4 °C
- PSA:94.83000
- Density:1.36 g/cm3
- LogP:1.84370
Flumethasone(Cas 2135-17-3) Usage
|
Description |
Flumethasone is a moderately potent difluorinated corticosteroid ester with anti-inflammatory, antipruritic and vasoconstrictive properties. Its mechanism of action is thought to inhibit arachidonic acids role in the biosynthesis of prostaglandins and leukotrienes. Flumethasone is active when it is not bound to the plasma protein transcortin. The drug flumethasone can be used to treat a variety of skin conditions, such as contact dermatitis, bug bites and eczema. |
|
Uses |
Flumethasone is an anti-inflammatory glucocorticoid used in veterinary practice and used as an anti-inflammatory for a variety of animals. It is commonly investigated for its effectiveness in animals to combat inflammatory symptoms caused by pathogens and to better understand inflammatory mechanisms. It is also used as to investigate novel techniques that detect and quantify steroid compounds within muscles. For the treatment of contact dermatitis, atopic dermatitis, exczema, psoriasis, diaper rash and other skin conditions. Flumethasone is a corticosteroid for topical use, in combination with Clioquinol for the treatment of otitis externa and otomycosis. Flumethasone shows fully 420 times the potency of cortisone in an animal model for anti-inflammatory activity. Flumethasone has been shown to exert anti-inflammtory effects by functioning as a glucocorticoid receptor agonist. Stimulating this receptor promotes inhibition of phospholipase A2 activity. This enzyme catalyzes arachidonic acid into eicosanoids most of which stimulate anti-inflammation mechanisms. |
|
Application |
Flumethasone is a topical corticosteroid of the glucocorticoid class used in the treatment of skin disorders where it reduces inflammation. Flumethasone is insoluble in water. Flumethasone pivalate is a topical difluorinated corticosteroid ester with anti-inflammatory, antipruritic and vasoconstrictive properties. A prompt decrease in inflammation, exudation and itching is experienced after application. It is commonly used in veterinary practice and has been used in cortisol assays to study early porcine conceptus development. |
|
Biological Activity |
Flumethasone pivalate is a glucocorticoid receptor agonist used in studies on plasma transcortin binding. It binds to the nucleus causing a variety of genetic activations and repressions. The anti-inflammatory action of flumethasone is thought to involve lipocortins and phospholipase A2 inhibitory proteins, which results in the inhibition of arachidonic acid and the control of prostaglandin and leukotriene biosynthesis. Flumethasone suppresses the immune system due to a decrease in the function of the lymphatic system, a reduction in immunoglobulin and complement concentrations, the precipitation of lymphocytopenia, and interference with antigen-antibody binding. |
|
Chemical Properties |
White Solid |
|
Originator |
Locacorten,Ciba,W. Germany,1964 |
|
Manufacturing Process |
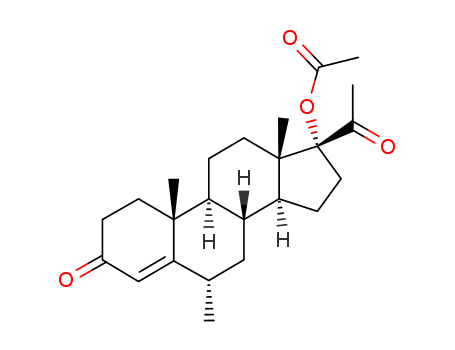
To approximately 1.3 g of hydrogen fluoride contained in a polyethylene bottle and maintained at -60°C was added 2.3 ml of tetrahydrofuran and then a solution of 500 mg (0.0012 mol) of 6α-fluoro-9β,11β-epoxy-16α-methyl- 17α,21-dihydroxy-1,4-pregnadiene-3,20-dione-21-acetate in 2 ml of methylenechloride. The steroid solution was rinsed in with an additional 1 ml of methylene chloride. The light red colored solution was then kept at approximately -30°C for 1 hour and at -10°C for 2 hours. At the end of this period it was mixed cautiously with an excess of cold sodium bicarbonate solution and the organic material extracted with the aid of additional methylene chloride. The combined extracts were washed with water, dried over anhydrous sodium sulfate and concentrated to approximately 35 ml. The solution was chromatographed over 130 g of Florisil anhydrous magnesium silicate. The column was developed with 260 ml portions of hexanes (Skellysolve B) containing increasing proportions of acetone. There was thus eluted 6α,9α-difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene- 3,20-dione 21-acetate which was freed of solvent by evaporation of the eluate fractions. |
|
Brand name |
Locorten (Novartis). |
|
Therapeutic Function |
Glucocorticoid, Antiinflammatory |
|
Veterinary Drugs and Treatments |
Flumethasone injection (Flucort?) is labeled in horses as indicated for: 1) Musculoskeletal conditions due to inflammation, where permanent structural changes do not exist, such as bursitis, carpitis, osselets and myositis. Following therapy an appropriate period of rest should be instituted to allow a more normal return to function of the affected part. 2) In allergic states such as hives, urticaria and insect bites. Flumethasone injection (Flucort?) is labeled in dogs as indicated for: 1) Musculoskeletal conditions due to inflammation of muscles or joints and accessory structures, where permanent structural changes do not exist, such as arthritis, osteoarthritis, the disc syndrome and myositis. In septic arthritis appropriate antibacterial therapy should be concurrently administered. 2) In certain acute and chronic dermatoses of varying etiology to help control the pruritus, irritation and inflammation associated with these conditions. The drug has proven useful in otitis externa in conjunction with topical medication for similar reasons. 3) In allergic states such as hives, urticaria and insect bites. 4) Shock and shock-like states, by intravenous administration. Flumethasone injection (Flucort?) is labeled in cats as indicated for certain acute and chronic dermatoses of varying etiology to help control the pruritus, irritation and inflammation associated with these conditions. Glucocorticoids have been used in an attempt to treat practically every malady that afflicts man or animal, but there are three broad uses and dosage ranges for use of these agents. 1) Replacement of glucocorticoid activity in patients with adrenal insufficiency, 2) as an antiinflammatory agent, and 3) as an immunosuppressive. Among some of the uses for glucocorticoids include treatment of: endocrine conditions (e.g., adrenal insufficiency), rheumatic diseases (e.g., rheumatoid arthritis), collagen diseases (e.g., systemic lupus), allergic states, respiratory diseases (e.g., asthma), dermatologic diseases (e.g., pemphigus, allergic dermatoses), hematologic disorders (e.g., thrombocytopenias, autoimmune hemolytic anemias), neoplasias, nervous system disorders (increased CSF pressure), GI diseases (e.g., ulcerative colitis exacerbations), and renal diseases (e.g., nephrotic syndrome). Some glucocorticoids are used topically in the eye and skin for various conditions or are injected intra-articularly or intra-lesionally. The above listing is certainly not complete. |
InChI:InChI=1/C22H28F2O5/c1-11-6-13-14-8-16(23)15-7-12(26)4-5-19(15,2)21(14,24)17(27)9-20(13,3)22(11,29)18(28)10-25/h4-5,7,11,13-14,16-17,25,27,29H,6,8-10H2,1-3H3/t11-,13?,14?,16+,17+,19+,20+,21+,22+/m1/s1
2135-17-3 Relevant articles
Synthesis method of steroid corticosteroid 21-hydroxyl side chain
-
Paragraph 0042-0044, (2020/03/06)
The invention belongs to the technical f...
A 17, 21 - double-hydroxy steroid derivatives of synthetic method
-
Paragraph 0042; 0043; 0045; 0047; 0072, (2018/04/02)
The invention relates to a method of pre...
Ring opening and fluoridation method and device of steroidal epoxy compound
-
Paragraph 0038; 0039; 0041, (2017/07/22)
The invention discloses a method of prep...
PROCESS FOR THE PREPARATION OF 17-DESOXY-CORTICOSTEROIDS
-
Page/Page column 20, (2012/02/05)
The present invention provides an improv...
2135-17-3 Process route
-
-
C25H36F2O5Si

-
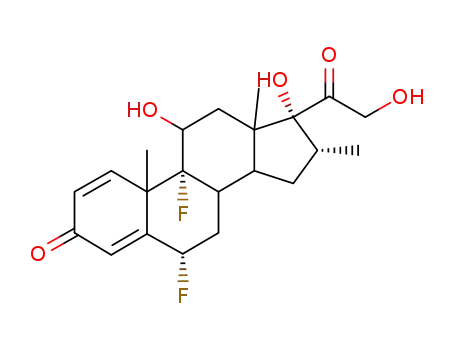
- 2135-17-3,2557-49-5,6477-56-1,59905-50-9,60895-22-9
6α-Fluor-dexamethason
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; water; pH=Ca. 1;
|
72.9 g |
-
-
C25H36F2O3Si

-

- 2135-17-3,2557-49-5,6477-56-1,59905-50-9,60895-22-9
6α-Fluor-dexamethason
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 3-chloro-benzenecarboperoxoic acid; potassium hydrogencarbonate / dichloromethane / 0 - 5 °C
2: hydrogenchloride / methanol; water / pH Ca. 1
With hydrogenchloride; potassium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid; In methanol; dichloromethane; water;
|
2135-17-3 Upstream products
-
2002-29-1
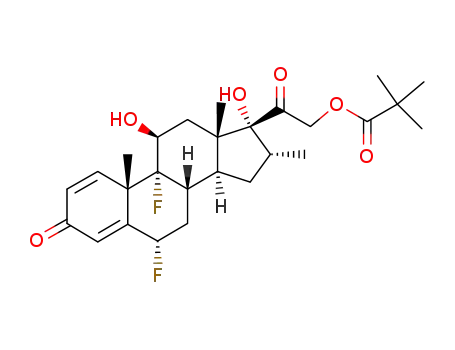
flumethasone pivalate
-
4571-51-1
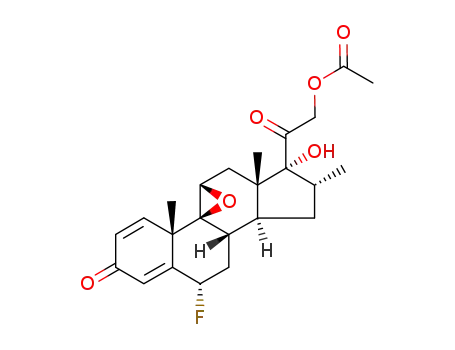
9β,11-epoxy-6α-fluoro-16α-methyl-17,21-dihydroxy-1,4-diene-3,20-dione 21-acetate
-
23961-95-7
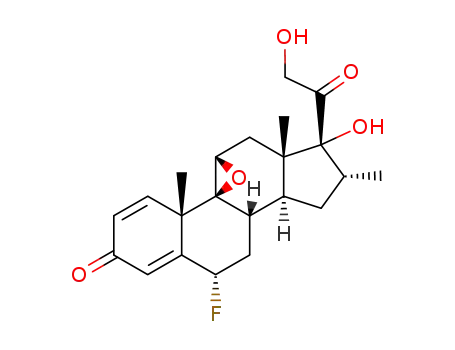
epoxyparamethasone
2135-17-3 Downstream products
-
28416-82-2
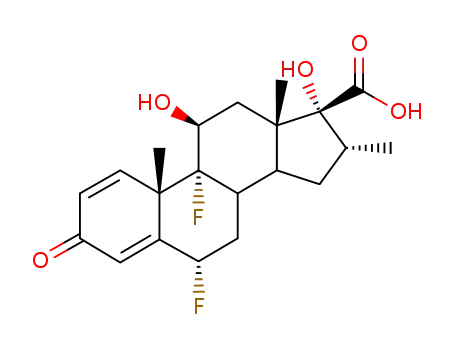
6α,9α-difluoro-11β,17α-dihydroxy-16α-methyl-3-oxo-androsta-1,4-diene-17β-carboxylic acid
-
25256-97-7
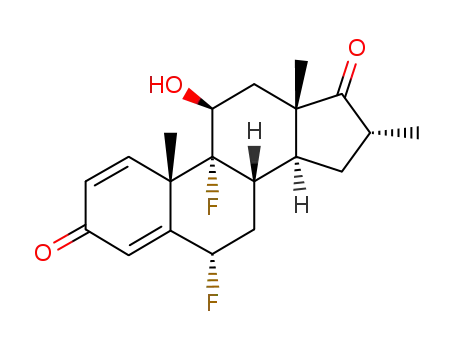
6α,9α-difluoro-16α-methyl-11β-hydroxyandrosta-1,4-dien-3-one
-
389119-99-7
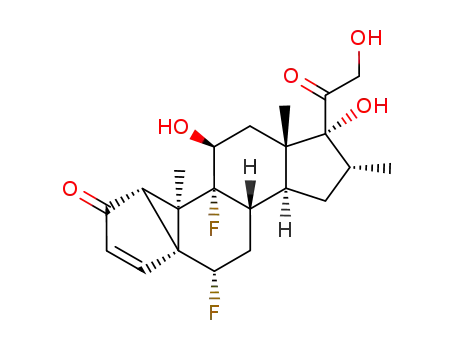
6α,9α-difluoro-11β,17α,21-trihydroxy-16α-methyl-1,5-cyclopregna-3-ene-2,20-dione
-
28416-82-2
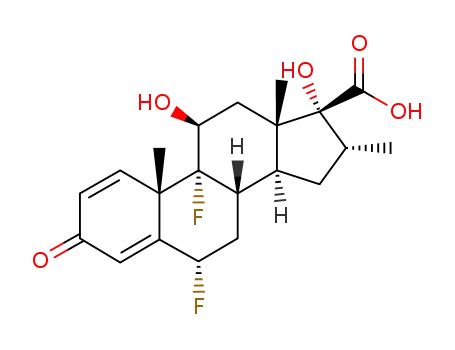
6α,9α-difluoro-11β,17α-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carboxylic acid
Relevant Products
-
Voriconazole
CAS:137234-62-9
-
Triamcinolone Acetonide
CAS:76-25-5
-
Lafutidine
CAS:118288-08-7