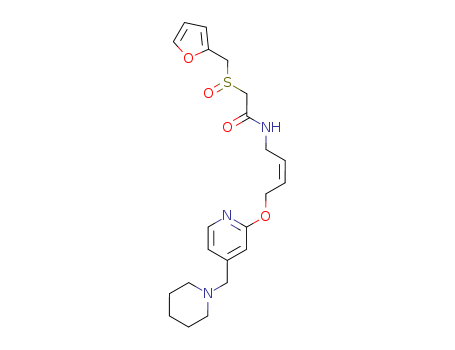
118288-08-7
- Product Name:Lafutidine
- Molecular Formula:C22H29N3O4S
- Purity:99%
- Molecular Weight:431.556
Product Details;
CasNo: 118288-08-7
Molecular Formula: C22H29N3O4S
Appearance: Yellowish white crystalline powder
Chinese Factory Supply Lafutidine 118288-08-7 Factory Sells with Reasonable Price
- Molecular Formula:C22H29N3O4S
- Molecular Weight:431.556
- Appearance/Colour:Yellowish white crystalline powder
- Vapor Pressure:1.12E-19mmHg at 25°C
- Melting Point:92.7-94.9°
- Refractive Index:1.598
- Boiling Point:704.2 °C at 760 mmHg
- PKA:13.13±0.46(Predicted)
- Flash Point:379.7 °C
- PSA:103.88000
- Density:1.252 g/cm3
- LogP:3.85510
Lafutidine(Cas 118288-08-7) Usage
|
Description |
Lafutidine is a second generation of H2-receptor antagonist. H2-receptor antagonist can strongly inhibit gastric acid secretion compared with conventional drugs such as antacids. Unlike conventional H2-receptor antagonist, lafutidine inhibits gastric acid secretion during daytime as well as nighttime in clinical studies in humans. Lafutidine also has gastroprotective activity independent of its acid antisecretory efficacy, preventing noxious agent-induced gastric mucosal injury and accelerating the repair process following gastric mucosal damage. It also protects experimentally induced reflux esophagitis, indomethacin-induced intestinal, and dextran sulfate sodium-induced colonic inflammation. Lafutidine is used for gastric and duodenal ulcers. It is also confirmed that lafutidine can be used as an empiric treatment and superior efficacy for primary care practice patients with dyspepsia. |
|
Originator |
Fujirebio (Japan) |
|
Uses |
(Z)-2-((Furan-2-ylmethyl)sulfinyl)-n-(4-((3-(piperidin-1-ylmethyl)pyridin-2-yl)oxy)but-2-en-1-yl)acetamide is a Histaminic H2 receptor antagonists in ulcer disease. Also, it is a model compound used to investigate the binding mechanism between antiulcer drugs and human serum albumin (HSA). |
|
Brand name |
Stogar, Protecadin |
InChI:InChI=1/C22H29N3O4S/c26-21(18-30(27)17-20-7-6-14-28-20)23-9-2-5-13-29-22-15-19(8-10-24-22)16-25-11-3-1-4-12-25/h2,5-8,10,14-15H,1,3-4,9,11-13,16-18H2,(H,23,26)/b5-2-
118288-08-7 Relevant articles
Increasing the Purity of Lafutidine Using a suicide Substrate
Wu, Chengjun,Li, Zhen,Wang, Chunchao,Zhou, Yanan,Sun, Tiemin
, p. 1081 - 1085 (2018)
When preparing lafutidine, we found that...
A novel histamine 2(H2) receptor antagonist with gastroprotective activity. II. Synthesis and pharmacological evaluation of 2-furfuryl-thio and 2-furfurylsulfinyl acetamide derivatives with heteroaromatic rings
Hirakawa, Nobuhiko,Matsumoto, Hajime,Hosoda, Akihiko,Sekine, Akihiro,Yamaura, Tetsuaki,Sekine, Yasuo
, p. 616 - 622 (1998)
We recently found that N-[3-[3- (piperid...
Method for manufacturing lafutidine crystal with high purity
-
Paragraph 0084; 0094-0104; 0118-0126, (2020/09/30)
The present invention relates to a metho...
Preparation method and application of lafutidine and intermediate thereof
-
Paragraph 0107-0108; 0111-0115; 0118-0122; 0125-0129; 0132, (2020/01/12)
The invention relates to a preparation m...
Lafutidine hydroxylamine hydrochloride method of preparation
-
Paragraph 0043-0044, (2016/10/08)
The invention relates to a novel chemica...
118288-08-7 Process route
-
![(2Z)-4-({4-[(piperidin-1-yl)methyl]pyridin-2-yl}oxy)but-2-en-1-amine](/upload/2023/9/7339380a-84c7-4538-b8c1-4bdc8e6da35b.png)
-
(2Z)-4-({4-[(piperidin-1-yl)methyl]pyridin-2-yl}oxy)but-2-en-1-amine

-
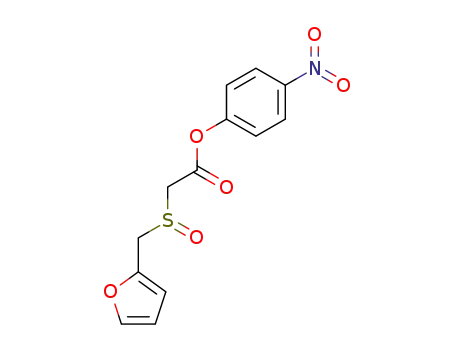
- 123855-55-0
p-nitrophenyl 2-(furfurylsulfinyl)acetate

-
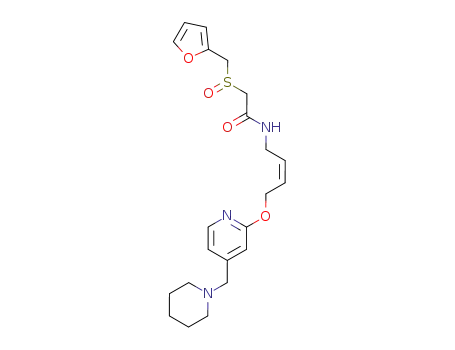
- 118288-08-7,206449-93-6,206449-94-7
lafutidine
| Conditions | Yield |
|---|---|
|
In dichloromethane; at 25 - 30 ℃;
|
94.6% |
|
In tetrahydrofuran; at 5 - 20 ℃; for 19h; Large scale;
|
91.3% |
|
In Isopropyl acetate; at 35 - 40 ℃; for 5h; Solvent;
|
87% |
|
In ethyl acetate; at 20 ℃;
|
60% |
-
![2-Bromo-4-[1,3]dioxolan-2-yl-pyridine](/upload/2023/9/f9120b4a-708b-4e52-8a21-6d43b63d1ce9.png)
- 118289-18-2
2-Bromo-4-[1,3]dioxolan-2-yl-pyridine

-

- 118288-08-7,206449-93-6,206449-94-7
lafutidine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 8 steps
1: 87 percent / NaOH, K2CO3, n-Bu4NHSO4 / toluene / 18 h / Ambient temperature
2: 90 percent / pyridinium p-toluenesulfonate, ethanol / 18 h / 55 °C
3: SOCl2, Et3N / CH2Cl2 / 1 h / 0 °C
4: n-Bu4NHSO4 / acetonitrile / 18 h / Heating
5: 78 percent / NH2NH2*H2O / methanol / 10 h / Heating
6: 91 percent / tetrahydrofuran / 18 h / Ambient temperature
7: 52 percent / p-TsOH, H2O / acetone / 18 h / Heating
8: 2.) NaBH4 / 1.) EtOH, 3 h, 2.) EtOH, 16 h
With sodium hydroxide; sodium tetrahydroborate; thionyl chloride; ethanol; water; tetra(n-butyl)ammonium hydrogensulfate; pyridinium p-toluenesulfonate; potassium carbonate; toluene-4-sulfonic acid; hydrazine hydrate; triethylamine; In tetrahydrofuran; methanol; dichloromethane; acetone; toluene; acetonitrile;
|
118288-08-7 Upstream products
-
110-89-4
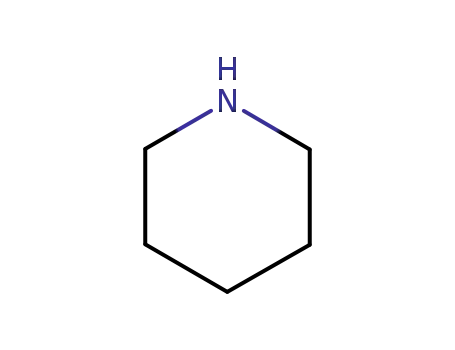
piperidine
-
118287-95-9
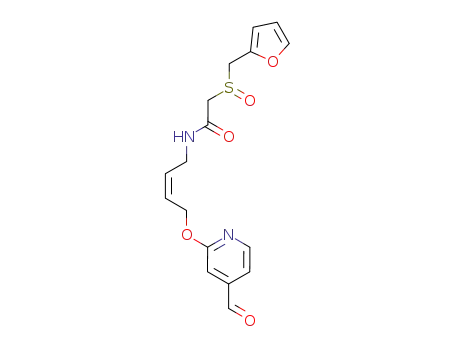
N-[(Z)-4-(4-Formyl-pyridin-2-yloxy)-but-2-enyl]-2-(furan-2-ylmethanesulfinyl)-acetamide
-
118289-18-2
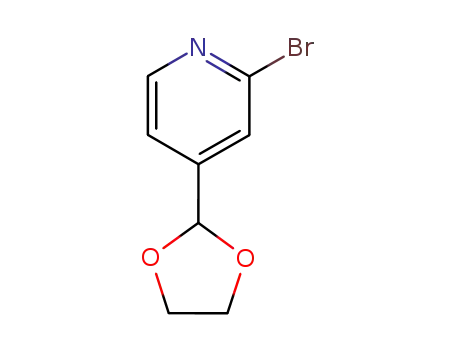
2-Bromo-4-[1,3]dioxolan-2-yl-pyridine
-
118289-22-8
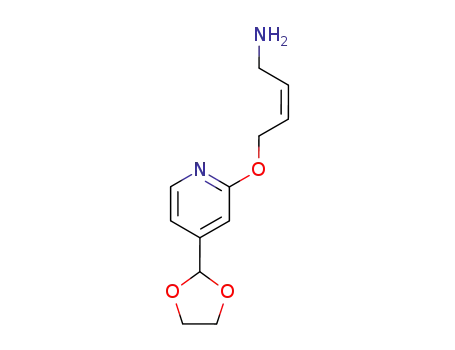
(Z)-4-(4-[1,3]Dioxolan-2-yl-pyridin-2-yloxy)-but-2-enylamine
Relevant Products
-
Febuxostat
CAS:144060-53-7
-
Flumethasone
CAS:2135-17-3
-
2-METHOXYCYCLOHEXANONE
CAS:7429-44-9