104344-23-2
- Product Name:Bisoprolol Fumarate
- Molecular Formula:C18H31NO4.C4H4O4
- Purity:99%
- Molecular Weight:766.97
Product Details;
CasNo: 104344-23-2
Molecular Formula: C18H31NO4.C4H4O4
Appearance: white to offf-white powder
Quality Factory Supply Reliable Quality Bisoprolol Fumarate 104344-23-2 Safe Shipping
- Molecular Formula:C4H4O4*2C18H31NO4
- Molecular Weight:766.97
- Appearance/Colour:white to offf-white powder
- Vapor Pressure:1.06E-08mmHg at 25°C
- Melting Point:100 °C
- Boiling Point:445 °C at 760 mmHg
- Flash Point:222.9 °C
- PSA:134.55000
- Density:1.033 g/cm3
- LogP:2.46860
Bisoprolol fumarate(Cas 104344-23-2) Usage
|
Pharmacological effects |
Bisoprolol fumarate is a selective β1-adrenergic receptor blockers. No intrinsic sympathomimetic activity and membrane stabilizing effect. Animal experiments of different models show that affinity for β1-receptor is 11 to 34-fold larger than β2-receptor. The selectivity for β1 receptor is four times of the similar drugs Atenolol. This product plays the role for a long time (24 hours or more), continual application takes good control of symptoms without tolerance phenomenon, with minimal side effects on the respiratory system, no effects on metabolism of fat. it also has a certain degree of block effect for bronchial β2 receptors, but it may only occur at high doses, usually it has no obvious clinical significance. |
|
Indications |
Treatment of essential hypertension. As first-line antihypertensive agents, it can be used alone or combined with diuretics and vasodilator drugs. Angina and myocardial infarction. Arrhythmias, such as the rapid ventricular arrhythmia, ventricular contraction. In recent years, bisoprolol fumarate is also tempted used for the treatment of heart failure. It is effective for moderate to severe chronic stable heart failure which previously receiving ACE inhibitors, diuretics and cardiac glycosides medications associated with ventricular systolic dysfunction (ejection fraction ≤35%) . |
|
Method and Dosage |
Hypertension: The initial dose is 5mg once, 1 times a day. It may be suitable for some patients to start with initial dose of 2.5mg (such as bronchial spasm disease). If the dose of 5 mg one day is not enough for antihypertensive effect , the dose may be increased to 10-20mg one day . Angina: initial dose of 2.5mg, 1 time a day, the maximum daily dose can not exceed 10mg. Heart failure: chronic, stable heart failure: it should start from small dose, if it is tolerated, the dose can gradually increasing (doubling the dose every 2-4 weeks) to the maximum tolerated dose or target dose. The maximum recommended target dose is 10mg one day , the starting dose is usually 1/8 of the target dose. |
|
Distinguish |
Take appropriate amount of this product powder (about the equivalent of bisoprolol fumarate 20mg), add 4ml water to dissolve, the filtrate is added 1 to 3 drops of potassium permanganate test solution , purple should fade , and brown precipitate forms. Take right amount of this product powder,it is dissolved in water and form the solution of which 1ml each contains about 0.1mg bisoprolol fumarate,after filtration, take continued filtrate, according to the UV-visible spectrophotometry (Appendix Ⅳ A) measure ,there is the maximum absorption in the 271nm wavelength. In the chromatogram recorded under the item of content determination ,the test solution peak retention time should be consistent with the reference solution peak retention time. |
|
Content Determine |
Take 10 tablets of this product , weigh accurately, porphyrize, accurately weigh appropriate amount (about the equivalent of bisoprolol fumarate 5mg), set it into 50ml volumetric flask, add water to make bisoprolol fumarate dissolve and dilute it to the mark , shake, filtrate, according to chromatographic conditions under the item of bisoprolol fumarate related substances,take the precise amount of continued filtrate 20μl into the liquid chromatograph, record the chromatograms; in addition , precisely weigh appropriate amount of the reference bisoprolol fumarate , dissolve it in water and dilute into the solution of which 1ml each contains about 0.1mg,measure in the same method, calculate according to the external standard method bisoprolol peak area ,it is obtained . |
|
Drug interactions |
1.when bisoprolol fumarate is used in combination with other anti-hypertensive drugs, the antihypertensive effect is enhanced . 2. when bisoprolol fumarate is used in combination with reserpine, methyldopa, clonidine or guanfacine, the heart rate can be slowed down. 3. when bisoprolol fumarate is used in combination with reserpine, reserpine needs to be stopped a few days after stop of this product . 4. when bisoprolol fumarate is used in combination with nifedipine, the antihypertensive effect of this product can be enhanced . 5. when bisoprolol fumarate is used in combination with verapamil or diltiazem ketones calcium icon antagonists or other antiarrhythmic drugs , there is the need for patient care, because they can cause hypotension, bradycardia and others. |
|
Precautions |
Diabetic patients with great blood glucose fluctuations or acidosis patients should be careful in use of it. Patients with pulmonary insufficiency, severe liver and kidney dysfunction should use with caution. Interruption of treatment should diminish doses daily ,when it is used in combination with other antihypertensive drugs, the dose often requires reductions. when drug overdose causes slow heartbeat or low blood pressure , patients must immediately stop taking this product. If necessary, take separate or sequential use of following drugs, atropine 0.5mg~2.0mg intravenously, appropriate amount of metaproterenol slow intravenous injection; glucagon 1mg~5mg (or 1mg~10mg). Because the antihypertensive effect of bisoprolol fumarate, capacity of the patient to drive or operate the machine may be weakened , particularly at the beginning of taking medication or conversion medication and taking with alcohol at the same time, but the person's ability to respond will not be directly affected . |
|
Side effects |
1.In early medication, mild fatigue, chest tightness, dizziness, bradycardia, drowsiness, palpitations, headache and lower limb edema may appear , and all automatically reduce or disappear after continuing taking . 2. In rare cases, there will be gastrointestinal disorders (diarrhea, constipation, nausea, abdominal pain) and skin reactions (eg erythema, itching). 3. Occasionally blood pressure decreases , pulse slow or atrioventricular conduction disorders. 4.Sometimes there will be tingling or cold extremities, in rare cases, it can cause muscle weakness, muscle cramps and less tears. 5.For patients with intermittent claudication or Raynaud's phenomenon,during the initial medication, the condition may worsen, the condition of existing myocardial dysfunction patients will possibly aggravate. 6.Occasionally airway resistance may increase . 7.Elderly patients with diabetes, their glucose tolerance may reduce , which may cover hypoglycemia manifestations (such as rapid heartbeat). |
|
Category |
Toxic substances |
|
Toxicity |
Moderate poisoning |
|
Acute toxicity |
Oral-rat LD50: 940 mg/kg; Oral-Mouse LD50: 678 mg/kg |
|
Flammability hazard characteristics |
Combustible; fire decomposition with toxic nitrogen oxide fumes |
|
Storage Characteristics |
Treasury temperature, ventilation, dry |
|
Extinguishing agent |
Water, carbon dioxide, dry, sandy soil |
|
Description |
Bisoprolol fumarate is a β1-selective adrenergic blocker useful in the treatment of essential hypertension. |
|
Chemical Properties |
White Solid |
|
Originator |
E. Merck (W. Germany) |
|
Uses |
antiinflammatory |
|
Manufacturing Process |
A solution of 10 g of 1-(p-2-isopropoxyethoxymethylphenoxy)-3-isopropylideneamino-propan-2-ol [obtainable by reacting 1-(p-2- isopropoxyethoxymethylphenoxy)-2,3-epoxy propane with ammonia to give 1- (p-2-isopropoxyethoxymethylphenoxy)-3-amino-propan-2-ol and subsequently reacting this with acetone] in 250 ml of ethanol was hydrogenated on 0.5 g of Raney nickel at 25°C under 1 atmosphere of pressure until 1 equivalent of H2 had been absorbed. The mixture was filtered and the filtrate evaporated to give 1-(p-2-iso-propoxyethoxymethyl-phenoxy)-3-isopropylamino-propan-2-ol, fumarate, m.p. 100°C (after addition of equimolecular quantity of fumaric acid). |
|
Brand name |
Zebeta (Duramed);CONCOR. |
|
Therapeutic Function |
Beta-adrenergic blocker |
|
Biological Activity |
A selective β 1 -adrenergic antagonist. Has a K d of 2-3 nM at the β 1 receptor and a β 1 / β 2 selectivity of approximately 100-fold. |
|
Biochem/physiol Actions |
Bisoprolol hemifumarate is useful in oral formulations due to its high bioavailability. It also shows long elimination half-life. |
|
Clinical Use |
Beta-1 adrenoceptor blocker: Hypertension Angina Adjunctive treatment for heart failure |
|
Metabolism |
Bisoprolol is excreted from the body by two routes. 50% is metabolised by the liver to inactive metabolites which are then excreted by the kidneys. The remaining 50% is excreted by the kidneys in an unmetabolised form. |
InChI:InChI=1/C18H31NO4.C4H4O4/c1-14(2)19-11-17(20)13-23-18-7-5-16(6-8-18)12-21-9-10-22-15(3)4;5-3(6)1-2-4(7)8/h5-8,14-15,17,19-20H,9-13H2,1-4H3;1-2H,(H,5,6)(H,7,8)/b;2-1+
104344-23-2 Relevant articles
Polymorphic forms of bisoprolol fumarate: Preparation and characterization
Detrich, ádám,D?m?t?r, Kata Judit,Katona, Miklós Tamás,Markovits, Imre,Vargáné Láng, Judit
, (2018/07/30)
Bisoprolol fumarate is a beta blocker-ty...
A NOVEL PROCESS FOR THE SYNTHESIS OF BISODPROLOL AND ITS INTERMEDIATE
-
Page/Page column 10, (2010/11/27)
This invention relates to a manufacturin...
Controlled release pharmaceutical preparation
-
, (2008/06/13)
A controlled release pharmaceutical prep...
104344-23-2 Process route
-
-
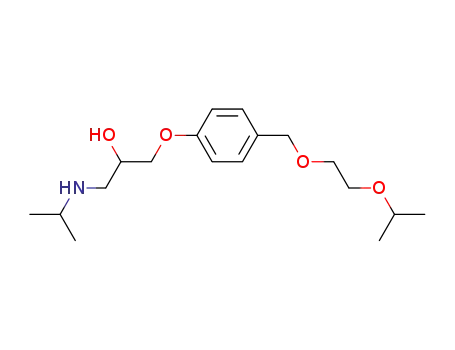
111051-40-2
bisoprolol

-
-
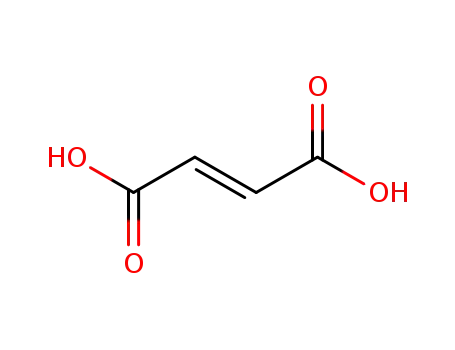
110-17-8,26099-09-2
(2E)-but-2-enedioic acid

-
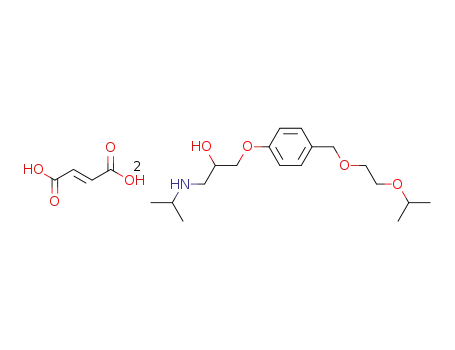
-
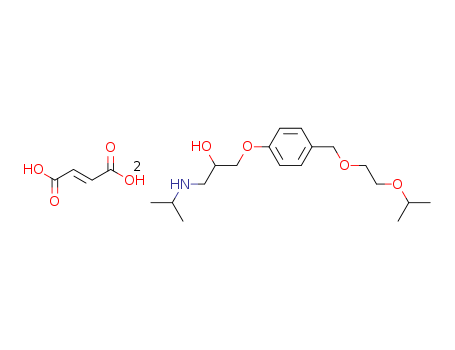
66722-45-0,103419-24-5,104344-23-2,105878-43-1,112945-47-8,208523-18-6
bisoprolol fumarate
| Conditions | Yield |
|---|---|
|
In
acetone;
for 0.25h;
Reflux;
|
85% |
|
In
acetone;
at 0 ℃;
for 1.5h;
Heating / reflux;
|

-

-
66722-45-0,103419-24-5,104344-23-2,105878-43-1,112945-47-8,208523-18-6
bisoprolol fumarate
| Conditions | Yield |
|---|---|
|
|
|
|
In
acetone;
at 0 ℃;
for 1.5h;
Purification / work up;
Heating / reflux;
|
104344-23-2 Upstream products
-
111051-40-2

bisoprolol
-
110-17-8

(2E)-but-2-enedioic acid
104344-23-2 Downstream products
-
109-59-1
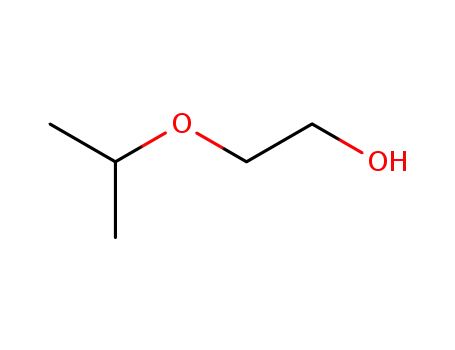
2-(1-methylethoxy)-ethanol
Relevant Products
-
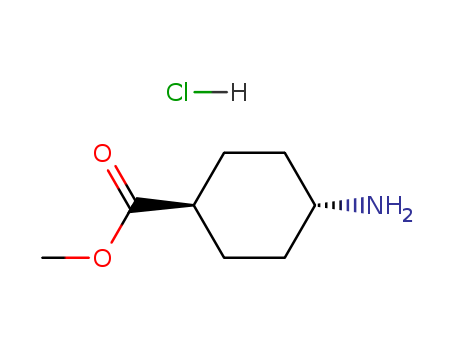
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
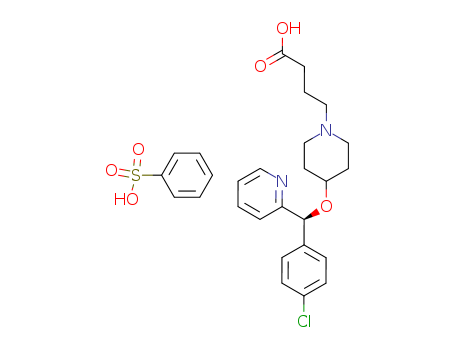
Bepotastine Besilate
CAS:190786-44-8
-
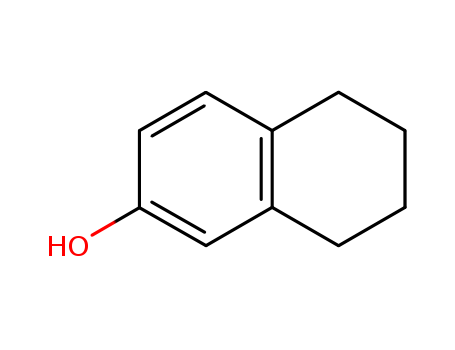
5,6,7,8-tetrahydro-2-naphthol
CAS:1125-78-6