1125-78-6
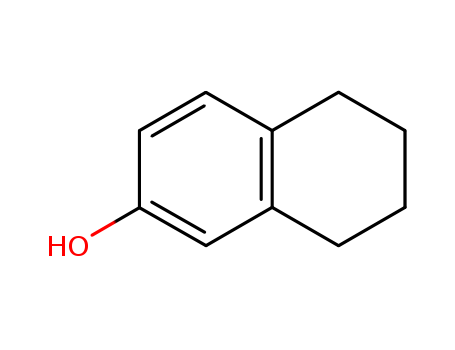
- Product Name:5,6,7,8-tetrahydro-2-naphthol
- Molecular Formula:C10H12O
- Purity:99%
- Molecular Weight:148.205
Product Details;
CasNo: 1125-78-6
Molecular Formula: C10H12O
Appearance: beige crystalline mass
Buy Reliable Quality 99% Pure 5,6,7,8-tetrahydro-2-naphthol 1125-78-6 Customized Supply
- Molecular Formula:C10H12O
- Molecular Weight:148.205
- Appearance/Colour:beige crystalline mass
- Melting Point:59-61 °C(lit.)
- Refractive Index:1.5000 (estimate)
- Boiling Point:275.5 °C at 760 mmHg
- PKA:10.48(at 25℃)
- Flash Point:131.8 °C
- PSA:20.23000
- Density:1.1 g/cm3
- LogP:2.27100
5,6,7,8-Tetrahydro-2-naphthol(Cas 1125-78-6) Usage
|
Chemical Properties |
beige crystalline mass |
|
Uses |
5,6,7,8-Tetrahydro-2-naphthol was used as a model compound in the study of photochemical transformation of 17β-estradiol (natural estrogenic steroid) and 17α-ethinylestradiol (synthetic oral contraceptive). |
|
Definition |
ChEBI: A member of the class tetralins that is 1,2,3,4-tetrahydronaphthalene which is substituted at position 6 by a hydroxy group. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 45, p. 5002, 1980 DOI: 10.1021/jo01312a044 |
InChI:InChI=1/C10H12O/c11-10-6-5-8-3-1-2-4-9(8)7-10/h5-7,11H,1-4H2
1125-78-6 Relevant articles
-
van Tamelen,Proost
, p. 3632 (1954)
-
-
Dauben,McKusick,Mueller
, p. 4179,4181 (1948)
-
Reduction of 1,1'-binaphthyls to octahydro-1,1'-binaphthyl derivatives with Raney Ni-Al alloy in aqueous solution
Guo,Ding
, p. 10061 - 10064 (2000)
Reduction of BINOL, BINAM and NOBIN with...
-
Adkins,Krsek
, p. 412 (1948)
-
-
Arnold,Evans
, p. 556 (1940)
-
-
Stork
, p. 576,578 (1947)
-
Partial ring hydrogenation of naphthols over supported metal catalysts in supercritical carbon dioxide solvent
Mine, Eiichi,Haryu, Eiji,Arai, Kunio,Sato, Takafumi,Sato, Osamu,Sasaki, Akiyoshi,Rode, Chandrashekhar V.,Shirai, Masayuki
, p. 782 - 783 (2005)
Selective ring hydrogenation of naphthol...
-
Shah,Tilak,Venkataraman
, p. 142,146 (1948)
-
-
Hunter,Nathan
, p. 2064 (1947)
-
Selective partial hydrogenation of hydroxy aromatic derivatives with palladium nanoparticles supported on hydrophilic carbon
Makowski, Philippe,Demir Cakan, Rezan,Antonietti, Markus,Goettmann, Frederic,Titirici, Maria-Magdalena
, p. 999 - 1001 (2008)
Selective hydrogenation of phenol to cyc...
HYDROGENATION AND HYDROGENOLYSIS. XVII. THE SELECTIVITIES OF PLATINUM GROUP METALS IN CATALYTIC HYDROGENATION OF 2-NAPHTHOL AND TETRAHYDRO-2-NAPHTHOLS.
Nishimura,Ohbuchi,Ikeno,Okada
, p. 2557 - 2564 (1984)
Catalytic hydrogenation of 2-naphthol (N...
A mild and practical method for deprotection of aryl methyl/benzyl/allyl ethers with HPPh2andtBuOK
Pan, Wenjing,Li, Chenchen,Zhu, Haoyin,Li, Fangfang,Li, Tao,Zhao, Wanxiang
, p. 7633 - 7640 (2021/09/22)
A general method for the demethylation, ...
Mild and Selective Rhodium-Catalyzed Transfer Hydrogenation of Functionalized Arenes
Wang, Yuhan,Chang, Zhiqian,Hu, Yan,Lin, Xiao,Dou, Xiaowei
supporting information, p. 1910 - 1914 (2021/03/08)
Diboron-mediated rhodium-catalyzed trans...
Tuning the Reactivity of Peroxo Anhydrides for Aromatic C-H Bond Oxidation
Pilevar, Afsaneh,Hosseini, Abolfazl,?ekutor, Marina,Hausmann, Heike,Becker, Jonathan,Turke, Kevin,Schreiner, Peter R.
, p. 10070 - 10079 (2018/09/06)
Phenol moieties are key structural motif...
Regioselective and Chemoselective Reduction of Naphthols Using Hydrosilane in Methanol: Synthesis of the 5,6,7,8-Tetrahydronaphthol Core
He, Yuan,Tang, Jinghua,Luo, Meiming,Zeng, Xiaoming
supporting information, p. 4159 - 4163 (2018/07/29)
A regioselective and chemoselective meth...
1125-78-6 Process route
-
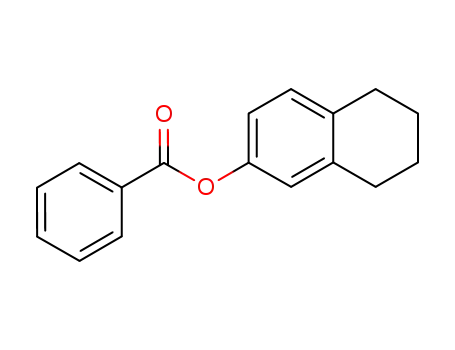
-
3574-36-5
5,6,7,8-tetrahydronaphthalen-2-yl benzoate

-
-
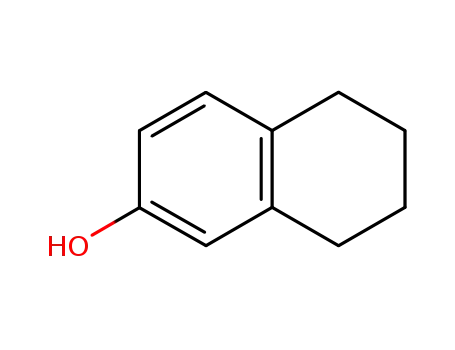
1125-78-6
5,6,7,8-Tetrahydro-2-naphthol

-
-
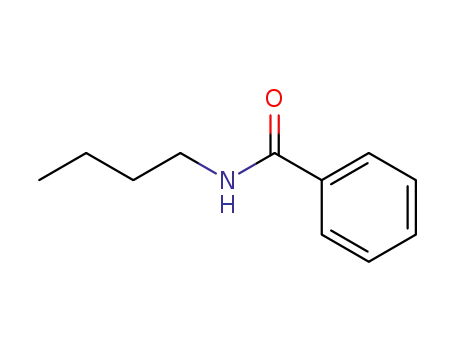
2782-40-3
N-butylbenzamide
| Conditions | Yield |
|---|---|
|
With
N-butylamine;
In
benzene;
for 22h;
Yield given;
Ambient temperature;
|
-

-
3574-36-5
5,6,7,8-tetrahydronaphthalen-2-yl benzoate

-
-
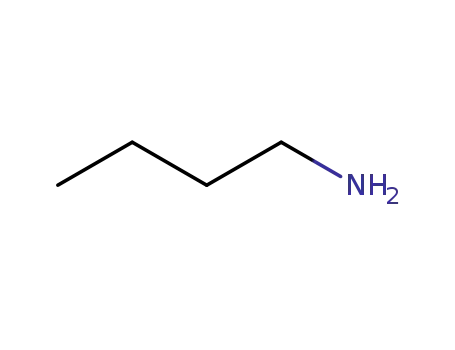
109-73-9,85404-21-3
N-butylamine

-

-
1125-78-6
5,6,7,8-Tetrahydro-2-naphthol

-

-
2782-40-3
N-butylbenzamide
| Conditions | Yield |
|---|---|
|
In
benzene;
for 22h;
Yields of byproduct given;
Ambient temperature;
|
1125-78-6 Upstream products
-
108-75-8
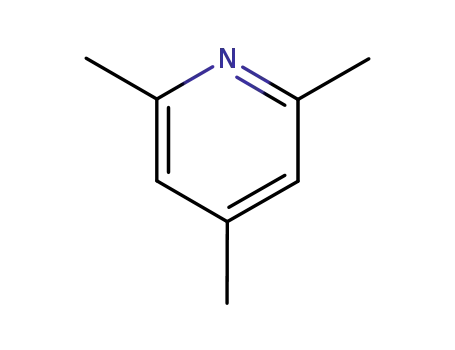
2,4,6-trimethyl-pyridine
-
76158-40-2
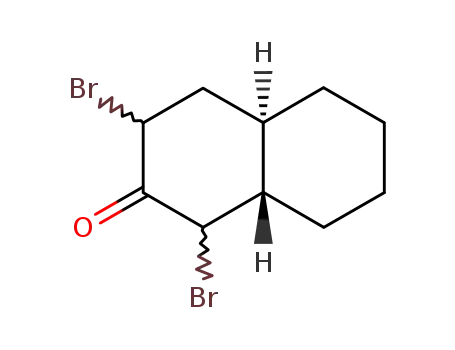
(+/-)-1ξ.3ξ-dibromo-2-oxo-(4arH.8atH)-decalin
-
91-22-5
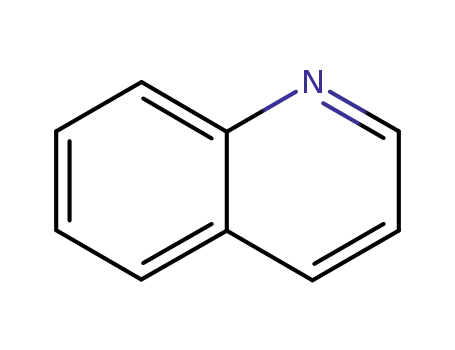
quinoline
-
100519-38-8
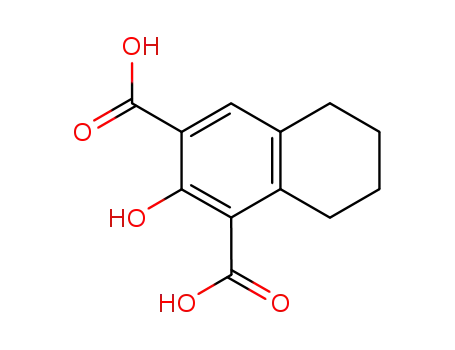
2-hydroxy-5,6,7,8-tetrahydro-naphthalene-1,3-dicarboxylic acid
1125-78-6 Downstream products
-
1730-48-9
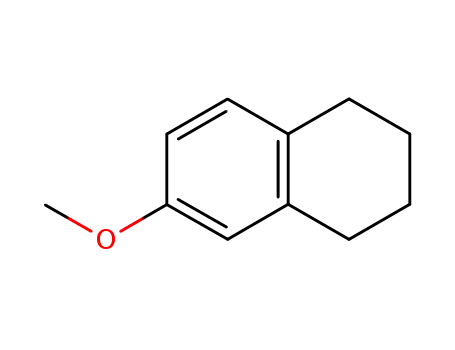
6-methoxy-1,2,3,4-tetrahydronaphthalene
-
1579-21-1
2-decalone
-
691892-19-0
(4ar,8ac)-decahydro-naphthalen-2ξ-ol
-
91-20-3
naphthalene
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
Bisoprolol Fumarate
CAS:104344-23-2
-
3-Hydroxyacetophenone
CAS:121-71-1