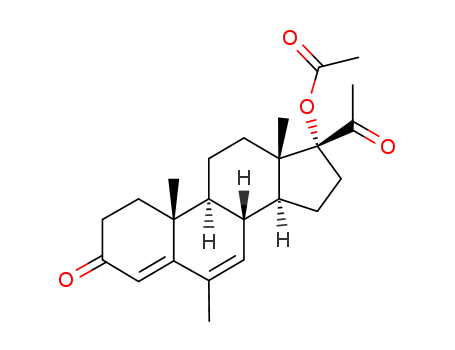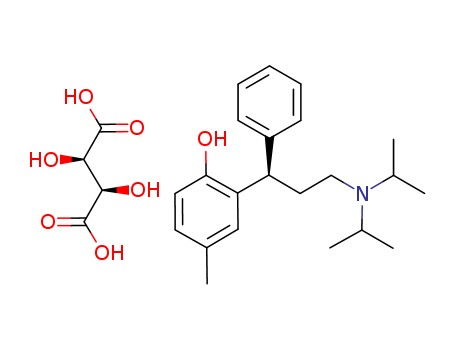
124937-52-6
- Product Name:Tolterodine Tartrate
- Molecular Formula:C22H31NO.C4H6O6
- Purity:99%
- Molecular Weight:475.582
Product Details;
CasNo: 124937-52-6
Molecular Formula: C22H31NO.C4H6O6
Appearance: white-to-off-white crystalline powder
Quality Factory Supply Sale Tolterodine Tartrate 124937-52-6 Cheap Price
- Molecular Formula:C22H31NO.C4H6O6
- Molecular Weight:475.582
- Appearance/Colour:white-to-off-white crystalline powder
- Vapor Pressure:1.97E-08mmHg at 25°C
- Melting Point:205-210 °C
- Boiling Point:442.2 °C at 760 mmHg
- PKA:9.87(at 25℃)
- Flash Point:192.1 °C
- PSA:138.53000
- Density:1.003 g/cm3
- LogP:3.21880
Tolterodine tartrate(Cas 124937-52-6) Usage
|
Chemical Properties |
White-to-Off-White Crystalline Powder |
|
Originator |
Detrol,Pharmacia and Upjohn |
|
Uses |
Tolterodine tartrate (Detrol LA) is a tartrate salt of tolterodine that is a competitive muscarinic receptor antagonist. Tolterodine tartrate (Detrol LA) is indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urge |
|
Manufacturing Process |
Trans-cinnamic acid (100 g, 675 mmol) is added to a 1 L 4-neck round bottom flask equipped with a mechanical stirrer, thermocouple, and nitrogen inlet. Para-cresol (76.6 g, 708 mmol) is preheated in a water bath at 60°C and added to the cinnamic acid followed by concentrated sulfuric acid (13.0 ml, 243 mmol). The reaction is immediately heated to a set point of 122.5°C and stirred at 120°-125°C until judged to be complete by HPLC analysis. When the reaction is complete the mixture is cooled to 100°C and added to a prewarmed separatory funnel (500 ml). The bottom layer containing the sulfuric acid is removed and toluene (280 ml), water (50 ml) and potassium carbonate (47%, 10 ml) are added to the separatory funnel containing the crude product. The pH of the aqueous layer is adjusted to between 5-8 with additional 47% potassium carbonate. The layers are separated and the organic layer is then washed once with water (50 ml). The organic layer is concentrated to a final volume of approximately 150 ml under reduced pressure. Isopropanol (350 ml) is then added, and distillation is continued to a volume of 350 ml. Isopropanol (150 ml) is again added and again distilled to 350 ml (2 times). The mixture is then cooled to 30°-40°C with rapid stirring until the product crystallizes. The rapid stirring is continued after crystallization. The product is cooled to 0°-5°C and held at this temperature for 1 h, filtered and washed with isopropanol (200 ml) cooled to 0°-5°C. If the last portion of the wash is colored the wash is continued until no more color is removed. The solids are then dried at 60°C under reduced pressure to give the 3,4-dihydro-6-methyl-4-phenyl-2H-benzopyran-2-one, melting point (uncorrected) 83°-85°C. 3,4-Dihydro-6-methyl-4-phenyl-2H-benzopyran-2-one (100.0 g, 420.2 mmol) is added to toluene (500 ml). The mixture is degassed by purging alternately with vacuum and nitrogen and then cooled to -21°C. Diisobutylaluminum hydride in toluene solution (DIBAL, 1.5 M, 290 ml, 435 mmol) is then slowly added over 2 h via add funnel while maintaining the reaction temperature at - 20°-25°C. The reaction is usually done when the DIBAL add is completed. If the reaction is not done additional DIBAL can be added in increments. When the reaction is done (<1% lactone) ethyl acetate (45 ml) is added at -20°- 25°C via add funnel. Very little exotherm is observed. Next, citric acid (23%, 500 ml) is added. The mixture is stirred at 45°-50°C for 1 h (or stirred overnight at 20°-25°C), the phases are separated, the organic phase is washed with water (2 times 300 ml). The organic phase is concentrated to 250 ml under reduced pressure. Methanol (500 ml) is added, and the mixture is concentrated to 250 ml. The methanol addition and distillation is repeated to give the 3,4-dihydro-6-methyl-4-phenyl-2H-benzopyran-2-ol in methanol solution. 3,4-Dihydro-6-methyl-4-phenyl-2H-benzopyran-2-ol in methanol (500 ml) is slowly added to palladium on carbon (5%, 22 g, 1.5 mmol) while maintaining a slight nitrogen purge. If 3,4-dihydro-6-methyl-4-phenyl-2H-benzopyran-2-ol is added too quickly without a nitrogen purge the catalyst will ignite the methanol. Diisopropylamine (147.0 ml, 1.05 mol) is added, and the mixture is hydrogenated at 45-50 psi and 48°C until the reaction is judged to be complete by HPLC (<2% lactol). The reaction is usually done after 10 h, but can be run overnight. The reaction mixture is cooled and removed from the hydrogenator using a methanol (150 ml) rinse. The combined reaction mixture and rinse is filtered through a bed of solka floc (10 g). The solka floc is washed thoroughly with methanol (100 ml) and the filtrate is concentrated to remove methanol while ethyl acetate is being added back. The volume of this solution of the (2-diisopropylamino)ethyl)benzyl)-p-cresol is adjusted to 700 ml using ethyl acetate and the mixture is heated to 55°C. To form the hydrochloride salt of the (2-diisopropylamino)ethyl)benzyl)-p_x0002_cresol (Tolterodine), concentrated hydrochloric acid (52.5 ml, 630 mmol) is added over 15 min. The resulting slurry is gradually cooled to -15°-20°C and held at this temperature for 1 h. Tolterodine hydrochloride is collected by filtration, washed 3 times with ethyl acetate, and dried overnight under reduced pressure at 600 to give the tolterodine hydrochloride, melting point 199°-201°C. Tolterodine hydrochloride (130.0 g, 359 mmol), methylene chloride (1.3 L) and water (650 ml) are mixed. The mixture is stirred rapidly while adding sodium hydroxide (50%, 13.0 ml) and sodium carbonate (13.0 g, 123 mmol). The pH as determined by pH paper is 10-11. After stirring thoroughly for approximately 15 min two clear homogeneous phases form. Stirring is continued for another 45 min, the layers are separated and the organic phase is washed with water (2 times 650 ml). The methylene chloride mixture is concentrated under reduced pressure. The concentrate is dissolved in ethanol (325 ml) and warmed to 60°-70°C. L-tartaric acid (80.84 g, 539 mmol) slurried in hot ethanol (810 ml) is added via add funnel at 60°-70°C over approximately 30 min. When the addition is done the slurry is refluxed for 1 h, gradually cooled to 0°C and held at this temperature for 1 h. The slurry is filtered, washed with ethanol (2 times 260 ml) previously cooled to 0°C, and dried overnight under reduced pressure at 60°C to give the crude title compound. The crude product (136.0 g) and ethanol (5.44 L) are mixed and heated to 80°C for 30 min. The mixture is concentrated to half the initial volume by distilling 2.72 L of ethanol. The mixture is gradually cooled to 20°- 25°C over 1 h, placed in an ice bath, and held at 0°C for 1 h. The tolterodine L-tartrate is collected by filtration, washed with ethanol (2 times 272 ml ) previously cooled to 0°C, and dried overnight under reduced pressure at 60°C to give product. This procedure was repeated a second time on 81.0 g of once recrystallized tolterodine L-tartrate to give the optically active (+)-(R)-2-(α- (2-(diisopropylamino)ethyl)benzyl)-p-cresol L-tartrate, melting point (uncorrected)=210°-211°C. |
|
Therapeutic Function |
Anticholinergic |
|
General Description |
Tolterodine Tartrate is a tertiary muscarinic?antagonist, basically used to manage urinary frequency, urgency, and incontinence in detrusor instability. |
|
Biological Activity |
tolterodine tartrate (detrol la) is a tartrate salt of tolterodine that is a competitive muscarinic receptor antagonist. |
|
Clinical Use |
Selective vasopressin V2 -receptor antagonist: Treatment of hyponatraemia secondary to SIADH To slow the progression of autosomal dominant polycystic kidney disease (ADPKD) |
|
Drug interactions |
Potentially hazardous interactions with other drugs Anti-arrhythmics: increased risk of ventricular arrhythmias with amiodarone, disopyramide and flecainide; increased risk of antimuscarinic side effects with disopyramide. Antifungals: avoid concomitant use with itraconazole and ketoconazole. Antivirals: avoid concomitant use with fosamprenavir, indinavir, lopinavir, ritonavir and saquinavir. Beta-blockers: increased risk of ventricular arrhythmias with sotalol. |
|
Metabolism |
Metabolised mainly by the cytochrome P450 isoenzyme CYP3A4. Eliminated mainly by the faecal route |
InChI:InChI=1/C22H31NO.C4H6O6/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24;5-1(3(7)8)2(6)4(9)10/h6-12,15-17,20,24H,13-14H2,1-5H3;1-2,5-6H,(H,7,8)(H,9,10)/t20-;/m1./s1
124937-52-6 Relevant articles
Ligand-Phospholipid Conjugation: A Versatile Strategy for Developing Long-Acting Ligands That Bind to Membrane Proteins by Restricting the Subcellular Localization of the Ligand
Kawamura, Shuhei,Ito, Yoshihiko,Hirokawa, Takatsugu,Hikiyama, Eriko,Yamada, Shizuo,Shuto, Satoshi
supporting information, p. 4020 - 4029 (2018/05/07)
We hypothesized that if drug localizatio...
Computationally-Led Ligand Modification using Interplay between Theory and Experiments: Highly Active Chiral Rhodium Catalyst Controlled by Electronic Effects and CH–π Interactions
Korenaga, Toshinobu,Sasaki, Ryo,Takemoto, Toshihide,Yasuda, Toshihisa,Watanabe, Masahito
supporting information, p. 322 - 333 (2018/01/22)
A chiral ligand for the rhodium-catalyze...
PROCESS FOR THE PREPARATION OF N,N-DIISOPROPYL-3-(2-HYDROXY-5-METHYLPHENYL)- 3-PHENYL PROPYLAMINE AND ITS SALTS STARTING FROM A NOVEL INTERMEDIATE
-
Page/Page column 25-26, (2012/08/07)
The invention concerns an improved proce...
Process for the preparation of N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)- 3-phenyl propylamine and its salts starting from a novel intermediate
-
Page/Page column 11, (2012/07/28)
The invention concerns an improved proce...
124937-52-6 Process route
-
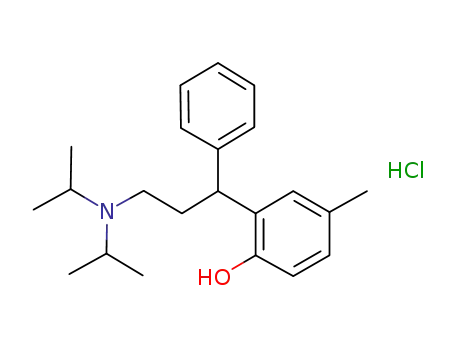
-
N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropylamine hydrochloride

-
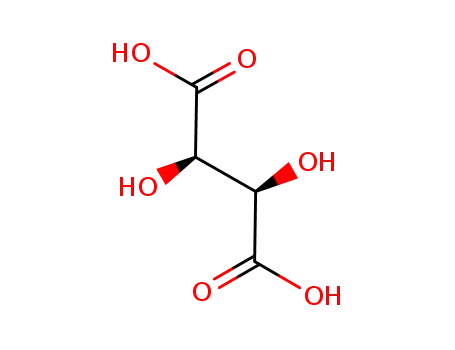
-
87-69-4,138508-61-9
L-Tartaric acid

-
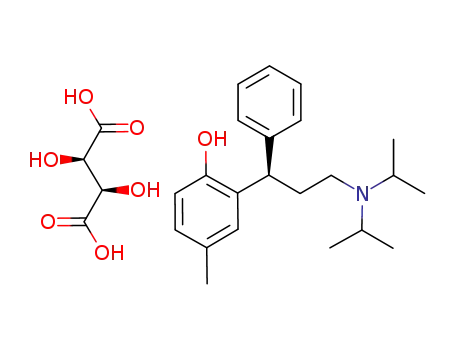
-
124937-52-6,209747-05-7
tolterodine tartrate
| Conditions | Yield |
|---|---|
|
N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropylamine hydrochloride;
With
sodium carbonate; sodium hydroxide;
In
dichloromethane; water;
at 20 ℃;
for 1h;
L-Tartaric acid;
In
methanol; acetone;
for 1h;
Reflux;
|
88% |
|
N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropylamine hydrochloride;
With
sodium carbonate; sodium hydroxide;
In
dichloromethane; water;
at 0 - 20 ℃;
for 1h;
L-Tartaric acid;
In
methanol; acetone;
at 0 ℃;
for 2h;
Reflux;
|
30% |
|
N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropylamine hydrochloride;
With
sodium hydroxide;
In
dichloromethane; water;
at 25 - 35 ℃;
for 0.0833333 - 0.166667h;
L-Tartaric acid;
In
methanol; dichloromethane; water; acetonitrile;
at 0 - 35 ℃;
for 6.58333 - 13h;
Heating / reflux;
|
|
|
N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropylamine hydrochloride;
With
sodium carbonate; sodium hydroxide;
In
dichloromethane; water;
L-Tartaric acid;
In
ethanol;
at 0 - 70 ℃;
|
99.2 % ee |
-
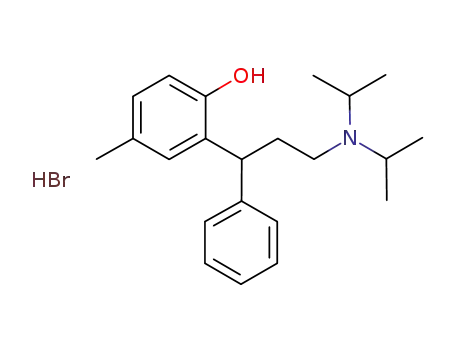
-
837376-36-0
3-(2-hydroxy-5-methylphenyl)-N,N-diisopropyl-3-phenylpropan-1-aminium bromide

-

-
87-69-4,138508-61-9
L-Tartaric acid

-

-
124937-52-6,209747-05-7
tolterodine tartrate
| Conditions | Yield |
|---|---|
|
3-(2-hydroxy-5-methylphenyl)-N,N-diisopropyl-3-phenylpropan-1-aminium bromide;
With
sodium hydroxide;
In
dichloromethane; water;
L-Tartaric acid;
In
ethanol;
at 60 - 85 ℃;
|
39% |
124937-52-6 Upstream products
-
87-69-4

L-Tartaric acid
-
124936-74-9
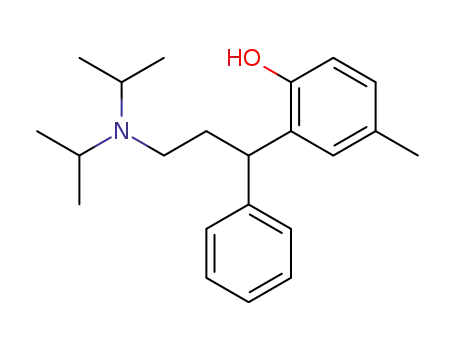
N,N-diiso-propyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropanamine
-
837376-36-0

3-(2-hydroxy-5-methylphenyl)-N,N-diisopropyl-3-phenylpropan-1-aminium bromide
-
124936-70-5
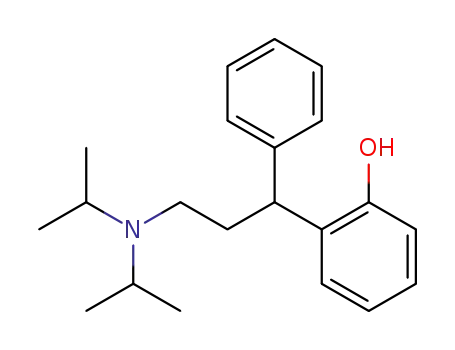
N,N-Diisopropyl-3-(2-hydroxyphenyl)-3-phenylpropylamine
124937-52-6 Downstream products
-
848768-06-9
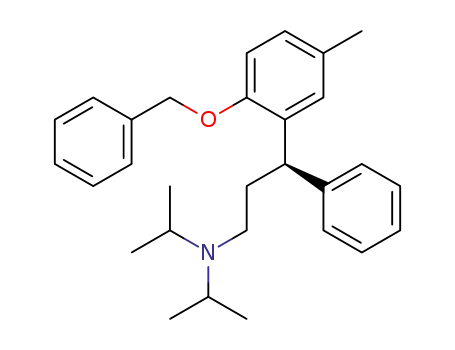
(R)-3-[2-(benzyloxy)-5-methylphenyl]-N,N-diisopropyl-3-phenylpropan-1-amine
-
74-98-6
propane
-
156755-37-2
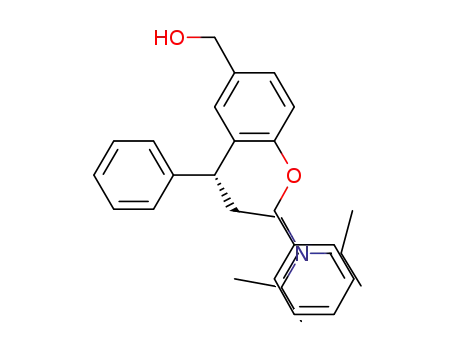
{4-(benzyloxy)-3-[(1R)-3-(dipropan-2-ylamino)-1-phenylpropyl]phenyl}methanol
-
214601-54-4
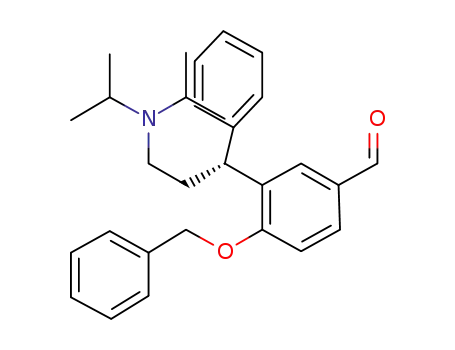
(R)-[4-benzyloxy-3-(3-diisopropylamino-1-phenyl-propyl)-phenyl]-methanal
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
2-[2-(2,2,2-trifluoroethoxy)phenoxy]-Ethanol,1-methanesulfonate
CAS:160969-03-9
-
Megestrol Acetate
CAS:595-33-5