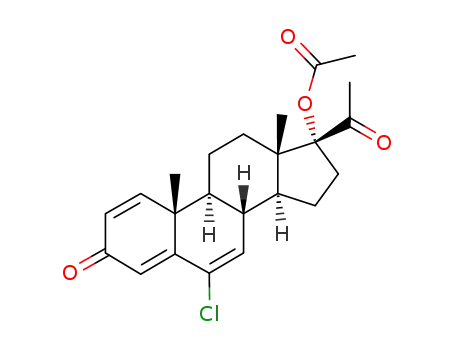
427-51-0
- Product Name:Cyproterone Acetate
- Molecular Formula:C24H29ClO4
- Purity:99%
- Molecular Weight:416.945
Product Details;
CasNo: 427-51-0
Molecular Formula: C24H29ClO4
Appearance: Crystalline solid
Factory Supply 99% Pure Cyproterone Acetate 427-51-0 Cheap Price
- Molecular Formula:C24H29ClO4
- Molecular Weight:416.945
- Appearance/Colour:Crystalline solid
- Vapor Pressure:3.78E-11mmHg at 25°C
- Melting Point:200-201 °C
- Refractive Index:1.582
- Boiling Point:525.9 °C at 760 mmHg
- Flash Point:177.6 °C
- PSA:60.44000
- Density:1.27 g/cm3
- LogP:4.60760
Cyproterone acetate(Cas 427-51-0) Usage
|
Description |
Cyproterone acetate (CPA) is an androgen receptor antagonist. It binds to human androgen receptors (Ki = 14 nM) and inhibits dihydrotestosterone-induced androgen receptor activation in CV-1 cells (IC50 = 26 nM). CPA (30 mg/kg) decreases ventral prostate weight in castrated immature rats. It also suppresses accessory sexual glands and fertility in adult male rats when administered at a dose of 10 mg/kg. CPA (0.3 μM) also induces apoptosis in primary adult female rat hepatocytes. Formulations containing cyproterone acetate have been used in the treatment of androgenization in females. |
|
Chemical Properties |
Crystalline Solid |
|
Originator |
Androcur ,Schering,W. Germany,1973 |
|
Uses |
Used as an antiandrogen. Combinded with estrogen in the treatment of acne |
|
Indications |
Cyproterone acetate is a progestational antiandrogen that blocks androgen receptor binding and suppresses androgen-sensitive tissues. It is available in a topical form in Europe for the treatment of hirsutism. |
|
Manufacturing Process |
2.34 g of 1,2α-methylene-δ4,6-pregnadiene-17α-ol-3,20-dione-17-acetate are dissolved in 18.25 cc of ethylene chloride which contains 844 rng of perbenzoic acid. The solution is stored for 16 hours at +5°C and 7 hours at room temperature. It is then diluted with methylene chloride and, with aqueous ferrous sulfate solution, sodium bicarbonate solution and with water washed until neutral.The organic phase is dried over sodium sulfate and then concentrated to dryness. 1.62 g of the thus obtained crude 1,2α-methylene-6,7α-oxido-δ4- pregnene-17α-ol-3,20-dione-17-acetate are dissolved in 109 cc of glacial acetic acid. This solution is then saturated at room temperature with hydrogen chloride gas and stored for 20 hours, It is then diluted with methylene chloride and washed with water until neutral.The organic phase is dried over sodium sulfate and then concentrated to dryness. The thus obtained crude 6-chloro-1α-chloromethyl-δ4,6-pregnadiene17α-ol-3,20-dione-17-acetate is heated to boiling in 20 cc of collidine for 20 minutes under nitrogen. After dilution with ether it is washed with 4 N hydrochloric acid and washed with water until neutral.After drying over sodium sulfate and concentration to vacuum the remaining residue is subjected to chromatography over silica gel. Using a benzene-ethyl acetate mixture (19:1) there is eluated 900 mg of 6-chloro-1,2α-methyleneδ4,6-pregnadiene-17α-ol-3,20-dione-17-acetate, which upon recrystallization from isopropyl ether melts at 200° to 201°C. |
|
Therapeutic Function |
Antiandrogen |
|
Clinical Use |
#N/A |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
Cyproterone is metabolised by various pathways including hydroxylation and conjugation; about 35% of a dose is excreted in urine, the remainder being excreted in the bile. The principal metabolite, 15β-hydroxycyproterone, has anti-androgenic activity |
InChI:InChI=1/C24H29ClO4/c1-12(26)24(29-13(2)27)8-6-16-14-10-20(25)19-11-21(28)15-9-18(15)23(19,4)17(14)5-7-22(16,24)3/h10-11,14-18H,5-9H2,1-4H3/t14?,15-,16?,17?,18+,22+,23+,24+/m1/s1
427-51-0 Relevant articles
Production technology and production device of c`yproterone acetate
-
Paragraph 0091; 0093, (2019/01/08)
The invention discloses a cyproterone ac...
Preparation process of cyproterone acetate (by machine translation)
-
, (2018/09/11)
The invention relates to a preparation p...
Further syntheses of cyproterone acetate
-
Page/Page column 3; 20; 30, (2010/02/07)
The present invention relates to improve...
KIT FOR FEMALE MAMMALS, COMPRISING A COMBINATION OF GESTAGEN AND OESTROGEN
-
, (2008/06/13)
-
427-51-0 Process route
-
-
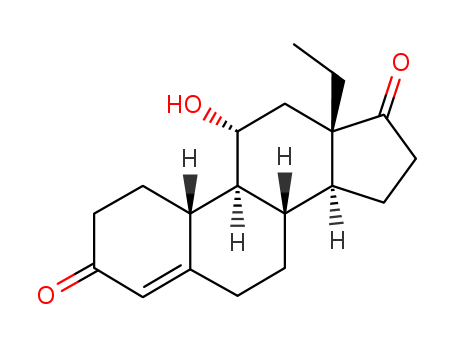
13698-49-2
6-chloro-17a-acetoxy-pregnane-1,4,6-,triene-3,20-dione

-
-
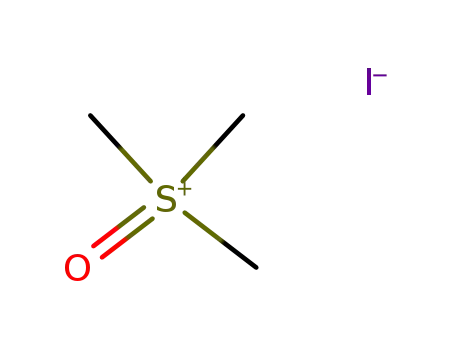
1774-47-6
trimethylsulfoxonium iodide

-
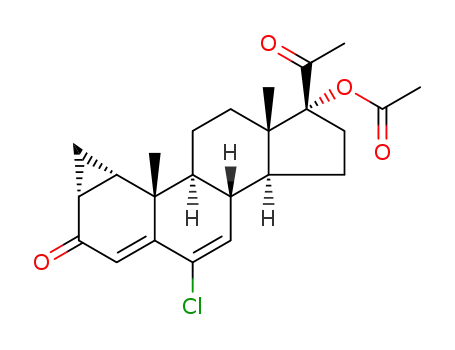
-
427-51-0
cyproterone acetate
| Conditions | Yield |
|---|---|
|
6-chloro-17a-acetoxy-pregnane-1,4,6-,triene-3,20-dione; trimethylsulfoxonium iodide;
With
sodium hydride;
In
dimethyl sulfoxide;
at 5 - 20 ℃;
for 29.5h;
With
hydrogenchloride;
In
water; dimethyl sulfoxide;
at 0 ℃;
|
51.51% |
-
-
C24H30Cl2O4

-

-
427-51-0
cyproterone acetate
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
In
methanol; N,N-dimethyl-formamide;
at 50 - 55 ℃;
for 5h;
|
427-51-0 Upstream products
-
13698-49-2

6-chloro-17a-acetoxy-pregnane-1,4,6-,triene-3,20-dione
-
1774-47-6

trimethylsulfoxonium iodide
-
17183-98-1
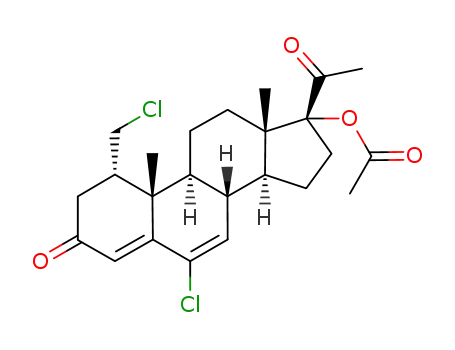
6-chloro-1α-chloromethyl-Δ4,6-pregnadien-17α-ol-3,20-dione-17-acetate
-
66212-25-7
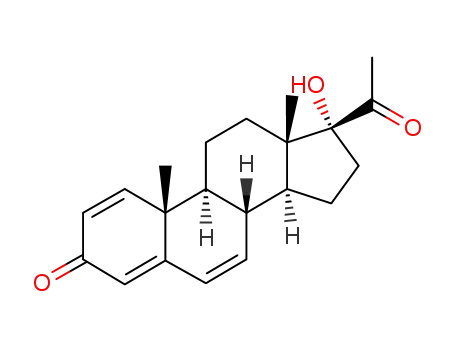
1,4,6-triene-3,20-dione-17α-hydroxyprogesterone
427-51-0 Downstream products
-
34554-28-4
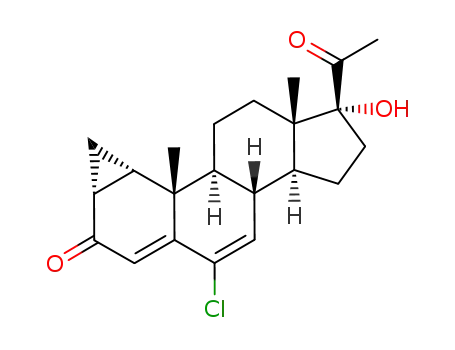
cyproterone
Relevant Products
-
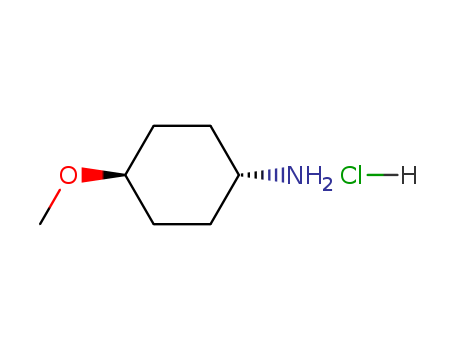
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
trans-4-MethoxycyclohexanaMine HCl
CAS:61367-41-7