630-56-8
- Product Name:Hydroxyprogesterone Caproate
- Molecular Formula:C27H40O4
- Purity:99%
- Molecular Weight:428.612
Product Details;
CasNo: 630-56-8
Molecular Formula: C27H40O4
Appearance: White or almost White Crystalline power
Chinese Manufacturer Supply Hydroxyprogesterone Caproate 630-56-8 Offer Fast Shipping
- Molecular Formula:C27H40O4
- Molecular Weight:428.612
- Appearance/Colour:White or almost White Crystalline power
- Vapor Pressure:1.4E-11mmHg at 25°C
- Melting Point:119 °C
- Refractive Index:1.533
- Boiling Point:536.4 °C at 760 mmHg
- Flash Point:227.6 °C
- PSA:60.44000
- Density:1.1 g/cm3
- LogP:5.96960
17a-Hydroxyprogesterone caproate(Cas 630-56-8) Usage
|
Description |
Hydroxyprogesterone caproate is a synthetic steroid hormone that is similar to medroxyprogesterone acetate and megestrol acetate. It is an ester derivative of 17α-hydroxyprogesterone formed from caproic acid (hexanoic acid). Hydroxyprogesterone caproate was previously marketed under the trade name Delalutin by Squibb, which was approved by the U.S. Food and Drug Administration (FDA) in 1956 and withdrawn from marketing in 1999. The U.S. FDA approved Makena from KV Pharmaceutical (previously named as Gestiva) on February 4, 2011 for prevention of preterm delivery in women with a history of preterm delivery, sparking a pricing controversy. |
|
Originator |
Delalutin,Squibb,US,1956 |
|
Uses |
Hydroxyprogesterone caproate is a synthetic progestin used for the prevention of spontaneous preterm births in singleton pregnancies in women who have previously had a spontaneous preterm birth. |
|
Manufacturing Process |
40 grams of 17α-oxypregnene-(5)-ol-(3)-one-(20)-acetate-(3) is brought to reaction with 22 grams of p-toluolsulfonic acid and 850 cc of caproic acid anhydride under a nitrogen atmosphere for 5 days at room temperature or 2,5 days at 37°C. The excess anhydride is blown off with steam in the presence of 200 cc of pyridine and the distillation residue is extracted with ether and worked up as usual. The remaining oil is brought to crystallization with pentane and the raw 17α-oxypregnenolone-3-acetate-17-caproate is recrystallized from methanol. The crystals are needle-like and have a MP of 104° to 105°C. This substance is partially saponified by refluxing for 1 hour in 1,800 cc of methanol in the presence of 13 cc of concentrated hydrochloric acid. After evaporation of the methanol under vacuum, the dry residue is recrystallized from isopropyl ether or methanol (dense needles). The thus obtained 17α-oxypregnenolone-17-caproate melts at 145° to 146.5°C. By oxidation in 100 cc of absolute toluol with 425 cc of cyclohexanone and 155 cc of a 20% aluminum isopropylate solution in absolute toluol and after repeated crystallizations from isopropyl ether or methanol, 24 grams of pure 17α-oxyprogesterone-17-caproate is obtained, MP 119° to 121°C (dense needles). |
|
Therapeutic Function |
Progestin |
|
General Description |
Hydroxyprogesteronecaproate, 17-hydroxypregn-4-ene-3,20-dionehexanoate, is much more active and longer acting than progesterone, probably because the 17αester hinders reduction to the 20-ol. In contrast, hydroxyprogesteroneitself lacks progestational activity. Thecaproate ester is given only intramuscularly. The estergreatly increases oil solubility, allowing it to be slowly releasedfrom depot preparations. Although only currently available throughcompounding pharmacies, a new formulation (Gestiva) wasdeemed approvable by the FDA in late 2006 for the preventionof preterm labor, pending further studies. |
|
Mechanism of action |
Hydroxyprogesterone caproate is a synthetic progestin. Hydroxyprogesterone is a potent, long-acting, progestational steroid ester which transforms proliferative endothelium into secretory endothelium, induces mammary gland duct development, and inhibits the production and/or release of gonadotropic hormone; it also shows slight estrogenic, androgenic, or corticoid effects as well, but should not be relied upon for these effects. It's mechanism for preventing preterm birth in women with a history of preterm delivery is unknown. |
|
Clinical Use |
Hydroxyprogesterone caproate (Makena?) is used to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. Hydroxyprogesterone caproate was designated an orphan drug by the US Food and Drug Administration (FDA) for this use in 2007. Efficacy of the drug for this use is based on improvement in the proportion of women who delivered at less than 37 weeks of gestation. Direct clinical benefit (e.g., improvement in neonatal morbidity and mortality) has not been established. While there are many risk factors for preterm birth, safety and efficacy of hydroxyprogesterone caproate have been demonstrated only in women with a prior spontaneous singleton birth. Hydroxyprogesterone is not intended for use in women with multiple gestations or other risk factors for preterm birth. The American College of Obstetricians and Gynecologists (ACOG) recommends that progesterone supplementation for the prevention of recurrent preterm birth be offered to women with a singleton pregnancy and a prior spontaneous preterm birth at less than 37 weeks of gestation due to spontaneous preterm labor or premature rupture of membranes. The ACOG also states that physicians should be able to prescribe Makena or compounded hydroxyprogesterone caproate based on accepted medical indications after discussion with the patient. |
|
Safety |
Originally marketed (Delalutin) nearly 50 years ago to prevent impending miscarriages, 17α-hydroxyprogesterone caproate was removed from the market in 2000 when its efficacy was called into question. 17α-Hydroxyprogesterone caproate, a synthetic injectable progesterone, was approved by the FDA in 2011 to reduce the risk of preterm delivery in women who have already experienced a preterm birth.It has not been shown to be effective in women carrying multiple fetuses.Injections begin between 16 and 21 weeks and are associated with pain, swelling, or itching at the injection site, hives, nausea, and diarrhea. Follow-up studies of the children born to women who used this drug indicate that developmental milestones were achieved at 2.5 and 5 years of age. A number of other pharmacologic agents are currently available for the prevention of preterm delivery including agents that can alter intracellular messengers (e.g., β-adrenergic receptor agonists, nitric oxide donors, magnesium sulfate and calcium channel blockers) and agents that modulate myometrial stimulants (e.g., inhibitors of prostaglandin synthesis and oxytocin antagonists). 17α-Hydroxyprogesterone caproate binds extensively to albumin and SHBG. From a metabolic perspective, it undergoes reduction, hydroxylation, and conjuga- tion reactions, including becoming glucuronidated, sulfated, and acetylated. It induces several cytochrome P450 (CYP) isozymes including CYP1A2, CYP2A6, and CYP2B6. |
InChI:InChI=1/C27H40O4/c1-5-6-7-8-24(30)31-27(18(2)28)16-13-23-21-10-9-19-17-20(29)11-14-25(19,3)22(21)12-15-26(23,27)4/h17,21-23H,5-16H2,1-4H3/t21?,22?,23?,25-,26-,27-/m0/s1
630-56-8 Relevant articles
Novel method for preparing progesterone caproate
-
Paragraph 0009-0011, (2021/10/05)
The invention discloses a novel method f...
Method for synthesis of progesterone-hexanoic acid (by machine translation)
-
Paragraph 0013-0015; 0017-0019, (2016/10/08)
The invention discloses a synthesis meth...
Preparation process of 17a-hydroxyprogesterone acetate
-
Paragraph 0031-0038; 0039-0046; 0047-0054, (2017/07/21)
The invention discloses a preparation pr...
THERAPEUTIC FOR HEPATIC CANCER
-
, (2011/02/18)
A novel pharmaceutical composition for t...
630-56-8 Process route
-
-
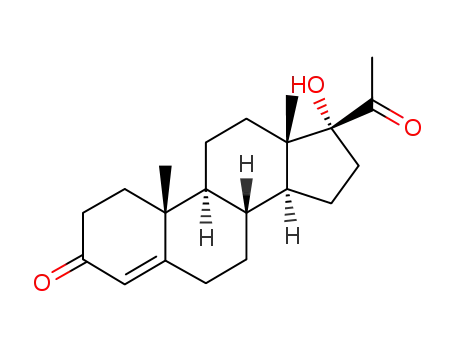
68-96-2
17-hydroxyprogesterone

-
-
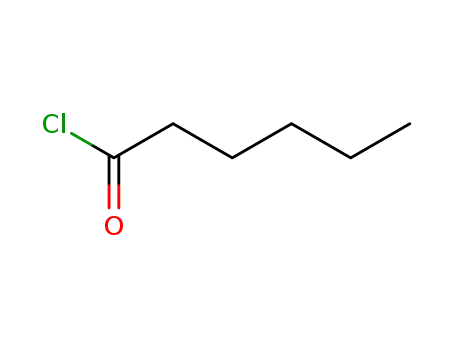
142-61-0
Hexanoyl chloride

-
-
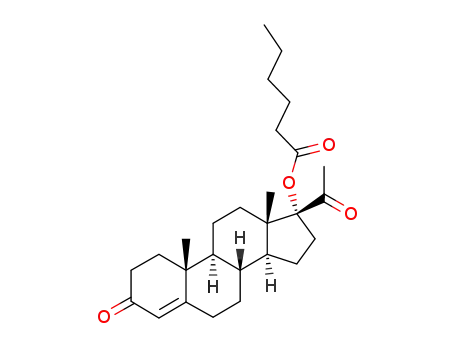
630-56-8
17-hexanoyloxy-pregn-4-ene-3,20-dione
| Conditions | Yield |
|---|---|
|
In
chloroform;
at 8 - 40 ℃;
for 5h;
Temperature;
|
91.4% |
-

-
68-96-2
17-hydroxyprogesterone

-
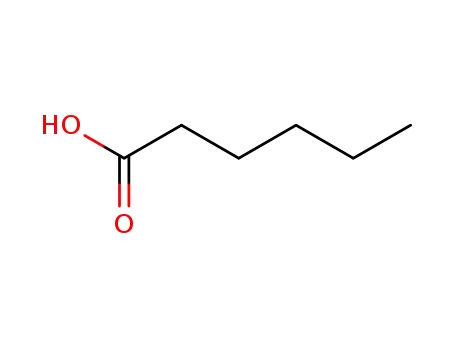
-
142-62-1
hexanoic acid

-

-
630-56-8
17-hexanoyloxy-pregn-4-ene-3,20-dione
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic anhydride;
|
|
|
17-hydroxyprogesterone; hexanoic acid;
With
pyridine; toluene-4-sulfonic acid;
In
toluene;
at 110 - 120 ℃;
for 3h;
With
hydrogenchloride;
In
ethanol; water;
for 2h;
Reagent/catalyst;
Solvent;
Reflux;
|
19.8 g |
630-56-8 Upstream products
-
83984-86-5
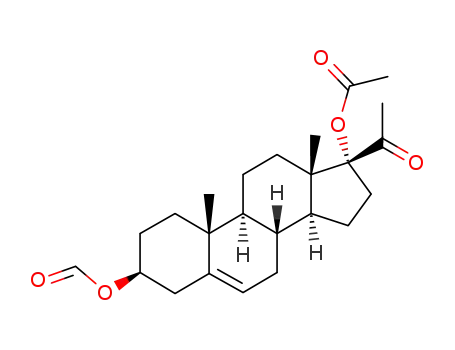
17-acetoxy-3β-formyloxy-pregn-5-en-20-one
-
68-96-2

17-hydroxyprogesterone
-
108-24-7
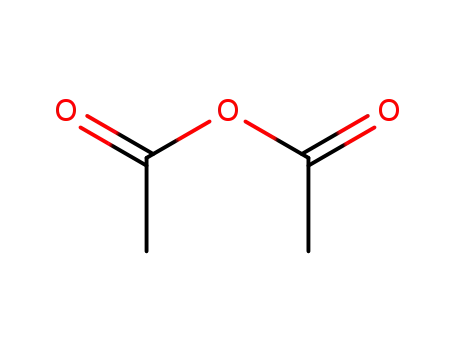
acetic anhydride
-
102752-45-4
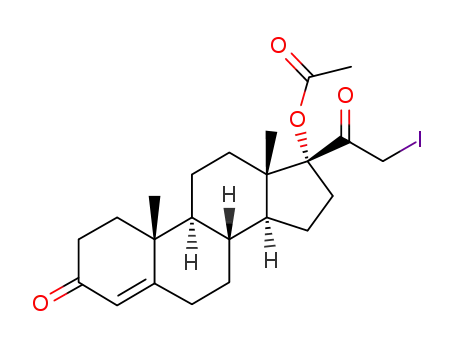
21-iodo-17α-hydroxypregn-4-ene-3,20-dione 17-acetate
630-56-8 Downstream products
-
68-96-2

17-hydroxyprogesterone
-
2668-74-8
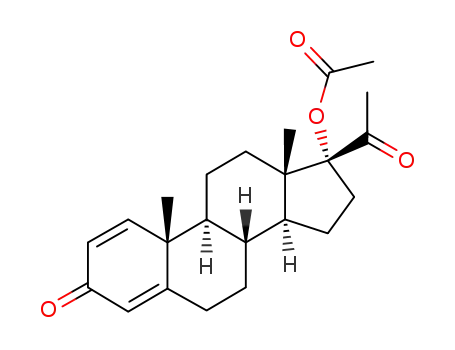
17-acetoxy-pregna-1,4-diene-3,20-dione
-
4954-07-8
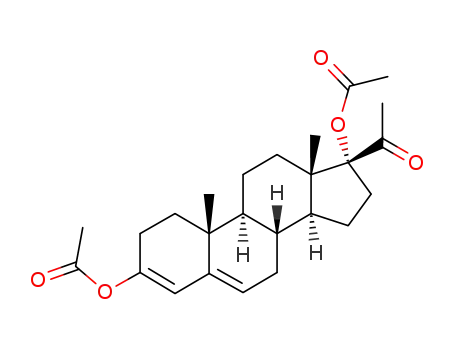
pregna-3,5-dien-20-one, 3,17-dihydroxy-, diacetate
-
32634-95-0
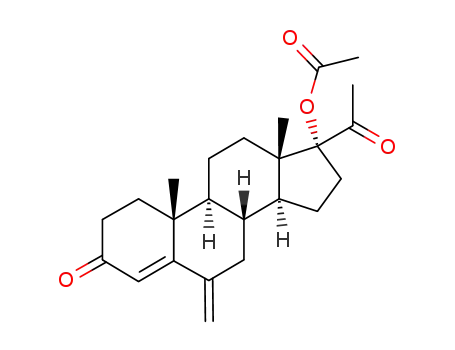
17α-Acetoxy-6-methylene-pregn-4-ene-3,20-dione
Relevant Products
-
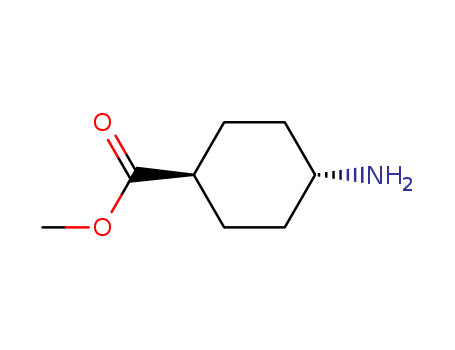
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
(1S,4S)-Methyl 4-aminocyclohexanecarboxylate
CAS:62456-15-9
-
ethyl 2-(4-hydroxyphenyl)-4-methyl-5-Thiazolecarboxylate
CAS:161797-99-5