152-62-5
- Product Name:Dydrogesterone
- Molecular Formula:C21H28O2
- Purity:99%
- Molecular Weight:312.452
Product Details;
CasNo: 152-62-5
Molecular Formula: C21H28O2
Factory Supply High Purity Top Purity Dydrogesterone 152-62-5 Safe Transportation
- Molecular Formula:C21H28O2
- Molecular Weight:312.452
- Vapor Pressure:9.55E-09mmHg at 25°C
- Melting Point:168-173 °C
- Refractive Index:1.556
- Boiling Point:462.8 °C at 760 mmHg
- Flash Point:172.2 °C
- PSA:34.14000
- Density:1.1 g/cm3
- LogP:4.49950
Dydrogesterone(Cas 152-62-5) Usage
|
Overview |
Dydrogesterone belongs to the "retrosteroid" class and is a stereoisomer of natural progesterone. This unique conformation is induced by exposing progesterone to ultraviolet light. This class of compounds was first described in the 1950s by Reerink et al. |
|
Properties |
Dydrogesterone is a synthetic progestin that does not have androgenic or estrogenic properties. Unlike some other progestational compounds, it does not cause an increase in body temperature and does not inhibit ovulation. |
|
Indications |
Dydrogesterone is used for the treatment of a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy[7]. |
|
Pharmacological Aspects |
Dydrogesterone is an orally active progestogen that acts directly on the uterus, which can produce a complete secretory endometrium in an estrogen-primed uterus. Dydrogesterone has no contraceptive effect as it does not inhibit or interfere with ovulation or the corpus luteum. Furthermore, dydrogesterone is non-androgenic, non-estrogenic, non-corticoid, non-anabolic and is not excreted as pregnanediol. Dydrogesterone can assist in regulating the healthy growth and normal shedding of the uterus lining. Therefore, it may be useful in the treatment of menstrual disorders such as absent, irregular or painful menstrual periods, infertility, premenstrual syndrome and endometriosis[3]. Dydrogesterone is an orally active 6-dehydro retroisomer of natural progesterone[fig. 1]. Dydrogesterone has progestational and antiestrogenic activity, but no significant estrogenic activity. Dydrogesterone is not chemically related to testosterone and its low affinity for the androgen receptor means that it has neither significant androgenic nor antiandrogenic activity[8]. In contrast, most 19-nortestosterone derivatives have androgenic activity[9] and thus tend to negate some of the beneficial effects of estrogen on lipid and lipoprotein levels[10] and to adversely affect glucose metabolism[11]. This does not occur with dydrogesterone. Dydrogesterone does not have significant glucocorticoid, antimineralocorticoid, and anti-inflammatory or thymolytic effects[12]. |
|
Pharmacodynamic profile |
Absorption and distribution Orally active, in contrast to natural progesterone[22] Tmax of dydrogesterone and its major metabolite DHD ~0.5 to 2.5 hours[23] Half-life of dydrogesterone ranges ~5 to 7 hours[23] Metabolism 43 metabolites identified[24] Most important metabolite is DHD[24] AUC for DHD is approximately 30to 40-fold greater than that of dydrogesterone after PO administration of dydrogesterone[24] Half-life~ of DHD ~14 to 17 hours[23] No aromatised metabolites, which accounts for the lack of estrogenic activity[24] No 17a-hydroxylation, which accounts for the lack of androgenic activity[24] Excretion Mainly excreted as metabolites in the urine[~63% of dose][24] Urinary excretion almost complete within 72 hours[24] |
|
Mechanisms of action |
Dydrogesterone is a kind of progestogens derivatives, sharing similar mechanism of action. Originally, progestogens were defined as compounds which maintain pregnancy, but, in the human, only progesterone can maintain pregnancy. The activity and potency of synthetic progestins are mostly evaluated by means of parameters associated with endometrial effects. The various actions of progesterone and synthetic progestins are brought about by genomic interactions with the progesterone receptors[PRs], which exist in the two isoforms, PRA and PRB, and by rapid non-genomic interactions with membrane binding sites. Moreover, according to their chemical structure, progestogens may bind to other members of the nuclear receptor superfamily. Binding of a progestogen to the receptor results in dimerization and interaction with a hormone responsive element within hormoneregulated target genes. In general, PRA may act as transcriptional repressor and PRB as activator. PRA may repress not only the transcriptional activity of the PRB, but also that of the ER, androgen receptor, and the glucocorticoid and mineralocorticoid receptors[25]. In most tissues, the biological action of progestogens is dependent on the presence of estrogens, as estrogens play a key role in the induction of PR, while progestogens down-regulate the expression of the ER. Progestogens may also reduce the expression of PR in the endometrial epithelium, but not the stromal and myometrial PR. Both PRA and PRB are expressed in the endometrial glands, whereas PRA predominates in the endometrial stroma[26]. In the breast of primates, progestogens may reduce the expression of the ERa and PR, but the estrogen-induced proliferation of the mammary epithelium is not inhibited, but enhanced by progestogens[27]. The primary role of progestogens in HRT is the inhibition of estrogen-induced proliferation of the endometrium. Moreover, they induce secretory changes in a proliferated endometrium. The antiestrogenic effect of progestogens in the endometrium is associated with a suppression of ER and the activation of the 17b-HSD type 2 which converts estradiol to estrone, and the estronesulfotransferase which causes conjugation of estrone. |
|
Adverse reactions |
Potential side effects of dydrogesterone may include pulsating headache which can develop into a migraine, pain in breast and breast tenderness, occasional blood spotting, pain during periods, irregular period, heavy bleeding during periods, missed period, weight gain, depression, abnormal liver function which can progress into, jaundice nausea and abdominal discomfort as well as hypersensitivity[28]. |
|
Precaution |
Following tips should be followed[28]: Patients should be monitored for visual disturbances, migraine headache or embolic disorders. The duration and the frequency of the treatment should be decided by the treating physician. If any allergic reaction reactions occur, the medicine should be stopped immediately and should consult a physician. Caution should be exercised in patients with a history of blood clot disorder, kidney impairment, heart disease, epilepsy, asthma, migraine, fluid retention, and breastfeeding. Monitor closely eye vision and symptoms of blood clot disorders. It may cause dizziness, do not drive a car or operate machinery while taking this medication. It should be used with extreme care in patients with a history of clinical depression. |
|
Uses |
Dydrogesterone is used in medical applications for various purposes. It is commonly employed for the treatment of irregular menstrual cycles and periods caused by progesterone deficiency. Additionally, it is used to prevent natural abortion in patients with a history of habitual abortions. |
|
Chemical Properties |
Light Yellow Solid |
|
Originator |
Duphaston,Duphar,UK,1961 |
|
Manufacturing Process |
A solution of 7.5 grams of retroprogesterone in 500 ml of freshly distilled tertiary butyl alcohol was refluxed with 12.75 grams of finely powdered chloranil, while stirring, for 5 hours in a nitrogen atmosphere. After cooling, 2 liters of water were added and extraction was performed three times with 200 ml of methylene dichloride. The combined extracts were then diluted with 1 liter of petroleum ether (40-60°C) washed successively with 100 ml of diluted Na2SO4, four times with 75 ml of 1 N NaOH, and then water to neutral reaction.By drying this solution on Na2SO4, and evaporating to dryness (last part in vacuo) 3.7 grams of crystalline residue was obtained. This residue was then dissolved in benzene. Filtration in benzene filtered through 35 grams of alumina (according to Brockmann was done and then the alumina was eluted with benzene. Evaporation of the benzene yielded 3.11 grams of crystalline residue. By crystallization with 15 ml of acetone at room temperature (at lower temperatures a by-product crystallized out) 900 mg of crystals, with a melting point of 165°-170°C were obtained. Transfer of the acetone mother liquor into a mixture of ethanol and hexane yielded 1.7 grams of a solid substance with a melting point of 130° to 145°C. This solid was then recrystallized with acetone at room temperature, yielding 600 mg of a solid with a melting point of 166° to 171°C. The two fairly pure fractions (600 mg and 900 mg) yielded, after crystallization with a mixture of acetone and hexane, finally 1.0 gram of 6-dehydroretroprogesterone, melting point 169° to 170°C. From the mother liquors an additional fraction of 0.44 gram with a melting point of 168° to 169°C was obtained. |
|
Brand name |
Gynorest (Solvay Pharmaceuticals). |
|
Therapeutic Function |
Progestin |
InChI:InChI=1/C21H28O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4-5,12,16-19H,6-11H2,1-3H3/t16-,17+,18-,19+,20?,21+/m0/s1
152-62-5 Relevant articles
Method and compound for synthetizing reprogestin
-
Paragraph 0083-0088; 0089-0092; 0093-0096, (2021/09/15)
To the synthesis method, dehydropregneno...
Method for synthesizing dydrogesterone
-
Paragraph 0029-0031, (2021/05/19)
The invention relates to a method for sy...
Production process capable of industrially synthesizing dydrogesterone
-
Paragraph 0090-0094, (2020/03/03)
The invention discloses a production pro...
PROCESS FOR PREPARATION OF DYDROGESTERONE
-
, (2018/09/08)
The present invention relates to process...
152-62-5 Process route
-
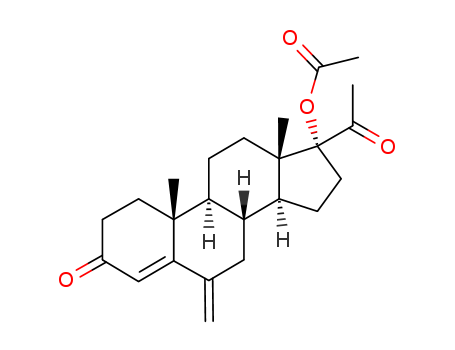
- 16484-70-1
7-Dehydro-9β,10α-progesteron

-
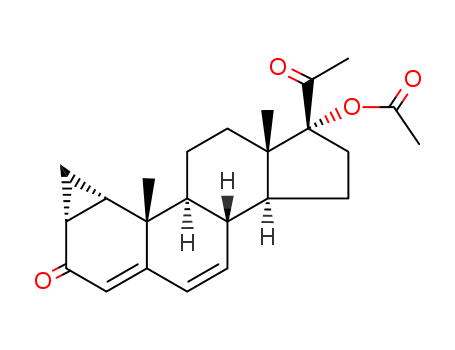
- 601-33-2,1162-56-7,2640-38-2,3795-24-2,25093-47-4,152-62-5
dydrogesterone
| Conditions | Yield |
|---|---|
|
With hydrogen bromide; acetic acid; at 0 ℃; Reagent/catalyst;
|
86.3% |
|
With hydrogenchloride; In tetrahydrofuran; water; at -5 ℃; Solvent; Temperature;
|
85% |
|
With hydrogenchloride; In water; isopropyl alcohol; at 10 ℃; for 2.5h; Reagent/catalyst; Solvent;
|
70% |
|
With hydrogenchloride; In isopropyl alcohol;
|
-
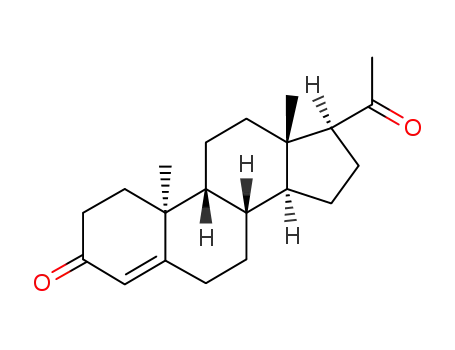
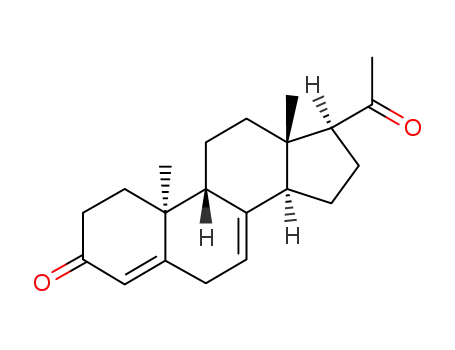
- 2755-10-4
(9β,10α)-pregn-4-ene-3,20-dione

-

- 601-33-2,1162-56-7,2640-38-2,3795-24-2,25093-47-4,152-62-5
dydrogesterone
| Conditions | Yield |
|---|---|
|
With chloranil; In tert-butyl alcohol; at 20 - 85 ℃; Reagent/catalyst; Temperature; Inert atmosphere;
|
152-62-5 Upstream products
-
16484-70-1

7-Dehydro-9β,10α-progesteron
-
10072-88-5
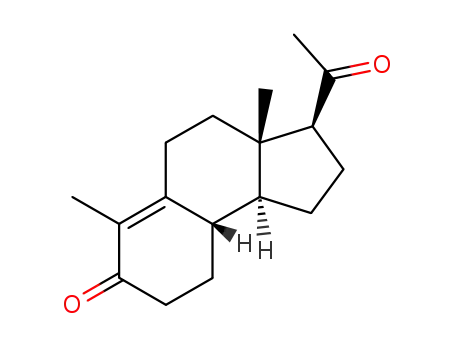
Des-A-17α-Δ9-pregnen-5,20-dion
-
2755-10-4

(9β,10α)-pregn-4-ene-3,20-dione
-
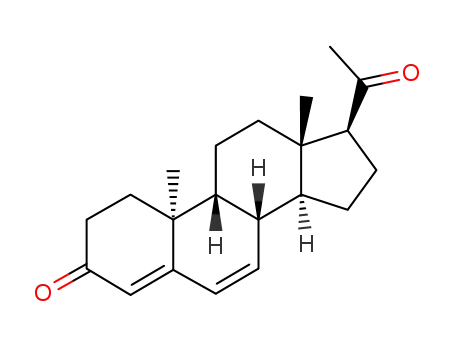
57-83-0
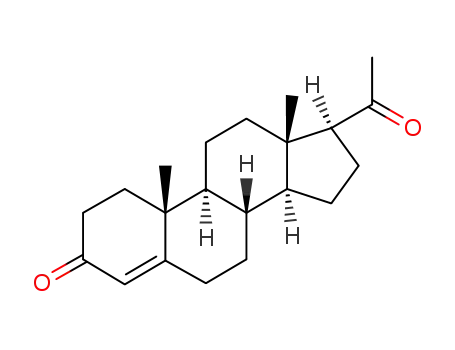
Progesterone
152-62-5 Downstream products
-
23035-53-2
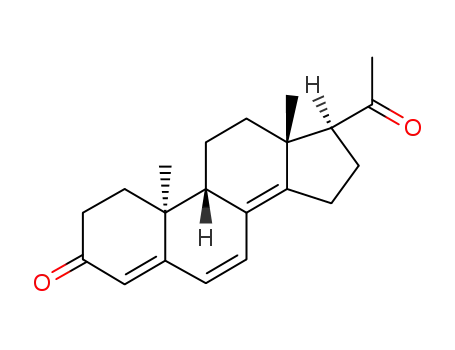
9β,10α-Pregna-4,6,8(14)-trien-3,20-dion
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
6-methylene-17α-hydroxyprogesterone acetate
CAS:32634-95-0