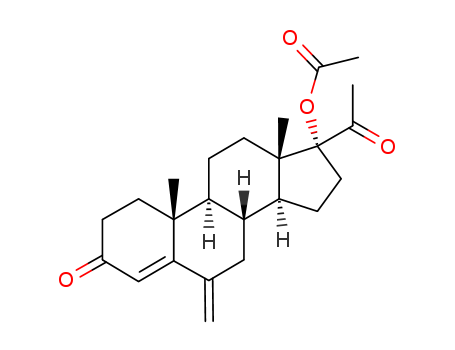
32634-95-0
- Product Name:6-methylene-17α-hydroxyprogesterone acetate
- Molecular Formula:C24H32O4
- Purity:99%
- Molecular Weight:384.516
Product Details;
CasNo: 32634-95-0
Molecular Formula: C24H32O4
Quality Manufacturer Supply 99% Pure 6-methylene-17α-hydroxyprogesterone acetate 32634-95-0 Fast Delivery
- Molecular Formula:C24H32O4
- Molecular Weight:384.516
- Vapor Pressure:2E-10mmHg at 25°C
- Melting Point:248-251℃ (methanol )
- Refractive Index:1.546
- Boiling Point:507.6 °C at 760 mmHg
- Flash Point:218.7 °C
- PSA:60.44000
- Density:1.14 g/cm3
- LogP:4.57530
6-methylene-17α-hydroxyprogesterone acetate (Cas 32634-95-0) Usage
| Description | 6-methylene-17α-hydroxyprogesterone acetate is an impurity of Medroxyprogesterone 17-Acetate. It can be prepared by reacting at 10°C for six hours. |
|
Uses |
6-methylene-17α-hydroxyprogesterone acetate is an impurity of Medroxyprogesterone 17-Acetate (M203560). Medroxyprogesterone 17-Acetate impurity F as per EP. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C24H32O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13,18-20H,1,6-12H2,2-5H3
32634-95-0 Relevant articles
Preparation method of 6-methylene-17alpha-hydroxyprogesterone acetate
-
Paragraph 0026; 0029; 0030; 0033, (2017/08/29)
The invention discloses a preparation me...
PROCESS FOR PREPARING 17ALPHA-ACETOXY-6-METHYLENEPREGN-4-ENE-3,20-DIONE, MEDROXYPROGESTERONE ACETATE AND MEGESTROL ACETATE
-
Page/Page column 3, (2009/01/24)
The present invention relates to a proce...
Novel 17 substituted pregnadiene derivatives as 5α-reductase inhibitors and their binding affinity for the androgen receptor
Cabeza, Marisa,Flores, Eugenio,Heuze, Ivonne,Sanchez, Mauricio,Bratoeff, Eugene,Ramirez, Elena,Francolugo, Victor Alfonso
, p. 535 - 539 (2007/10/03)
The in vitro antiandrogenic activity of ...
Molecular interactions of new pregnenedione derivatives.
Bratoeff, Eugene,Ramirez, Elena,Flores, Eugenio,Valencia, Norma,Sanchez, Mauricio,Heuze, Ivonne,Cabeza, Marisa
, p. 1132 - 1136 (2007/10/03)
The in vitro inhibitory activity of five...
32634-95-0 Process route
-
-
C26H39NO4

-
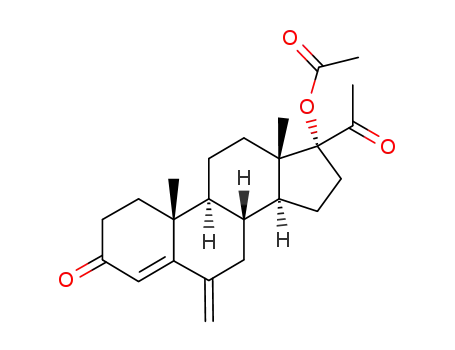
- 32634-95-0
17α-Acetoxy-6-methylene-pregn-4-ene-3,20-dione
| Conditions | Yield |
|---|---|
|
With phosphoric acid; In 1,4-dioxane; at 70 ℃; for 5h; Reagent/catalyst; Temperature;
|
98% |
-
-
C31H41NO4

-

- 32634-95-0
17α-Acetoxy-6-methylene-pregn-4-ene-3,20-dione
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; at 20 ℃; for 1h; Reagent/catalyst; Temperature;
|
98% |
32634-95-0 Upstream products
-
109-87-5
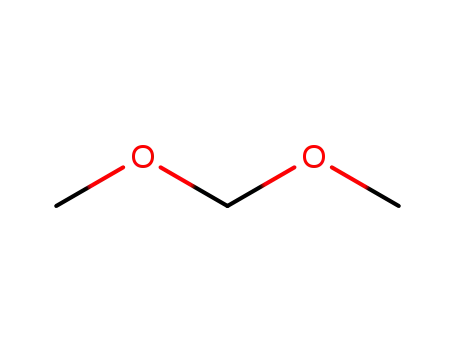
Dimethoxymethane
-
4134-58-1
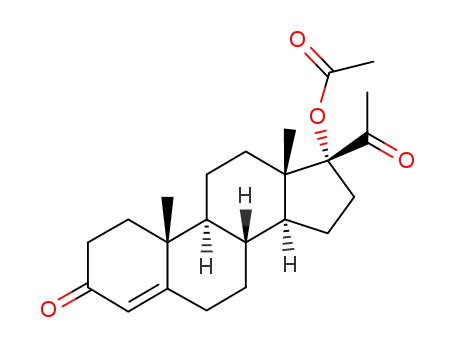
hydroxyprogesterone acetate
-
462-95-3
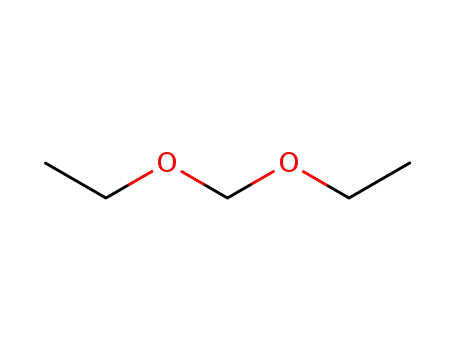
formaldehyde diethyl acetal
-
53476-47-4
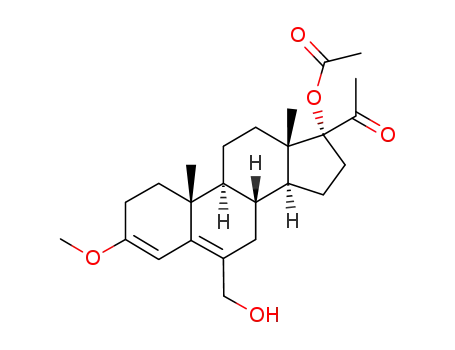
3-Methoxy-20-oxo-6-hydroxymethyl-17α-acetoxy-pregnadien-(3,5)
32634-95-0 Downstream products
-
595-33-5
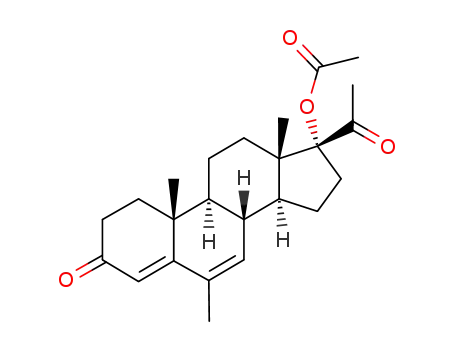
Megestrol acetate
-
71-58-9
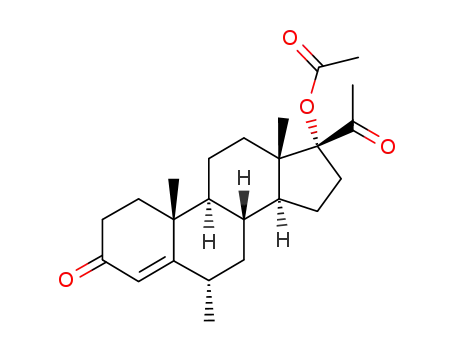
Medroxyprogesterone acetate
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
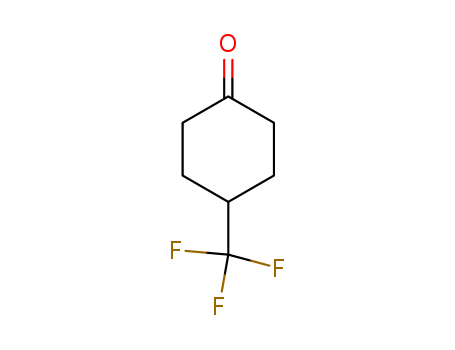
4-(trifluoromethyl)cyclohexanone
CAS:75091-99-5
-
Dydrogesterone
CAS:152-62-5