797-63-7
- Product Name:Levonorgestrel
- Molecular Formula:C21H28O2
- Purity:99%
- Molecular Weight:312.452
Product Details;
CasNo: 797-63-7
Molecular Formula: C21H28O2
Appearance: white solid
Factory Supply Hot Sale Levonorgestrel 797-63-7 Cheap Price
- Molecular Formula:C21H28O2
- Molecular Weight:312.452
- Appearance/Colour:white solid
- Vapor Pressure:2.32E-10mmHg at 25°C
- Melting Point:206 °C
- Refractive Index:1.571
- Boiling Point:459.1 °C at 760 mmHg
- PKA:13.09±0.40(Predicted)
- Flash Point:195.4 °C
- PSA:37.30000
- Density:1.13 g/cm3
- LogP:3.88260
Levonorgestrel(Cas 797-63-7) Usage
|
Overview |
Levonorgestrel (LNG) is a synthetic progestational hormone that has actions similar to those of progesterone and is approximately twice as potent as its racemic or ±-isomer norgestrel. Levonorgestrel is a levo-isomer of norgestrel and belongs to the progestin class of hormones. Progestins are synthetic compounds that have actions similar to the naturally occurring hormone progesterone. These hormones play a crucial role in the female reproductive system and are involved in various processes related to pregnancy and the menstrual cycle. Levonorgestrel can be administered in various forms, including orally, transdermally (as a patch combined with estradiol), and through an intrauterine device (IUD) for prolonged, continuous use. The oral bioavailability of levonorgestrel is approximately 95%. Levonorgestrel is considered one of the most effective and safe medicines by the World Health Organization and is available as a generic medication, making it widely accessible for contraception and emergency contraception purposes. |
|
Indication |
Levonorgestrel is a progestin-only emergency contraceptive indicated for the prevention of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. To obtain optimal efficacy, its should be taken as soon as possible within 72 hours of intercourse. In US, it(Plan B)?is available only by prescription for women younger than age 17 years, and available over the counter for women 17 years and older. However, it should be noted that it is not indicated for routine use as a contraceptive[7]. |
|
Pharmacokinetics |
Progesterone is rapidly absorbed following administration by any route. Its half-life in the plasma is approximately 5 minutes, and small amounts are stored temporarily in body fat. It is almost completely metabolized in liver to pregnanediol and conjugated with glucuronic acid. It is excreted into the urine as pregnanediol glucuronide. Most of the synthetic progestins are extensively metabolized to inactive products that are excreted mainly in the urine. Route of administration of levonorgestrel is oral. Duration of action is 1-3 days. It has Androgenic, Antiestrogenic and Anabolic activities[8]. The synthetic progestins have much longer half-lives, e.g., approximately 7 hours for norethindrone, 16 hours for norgestrel, 12 hours for gestodene, and 24 hours for MPA[6]. |
|
Pharmacodynamics |
Levonorgestrel is a synthetic form of the naturally occurring female sex hormone, progesterone[1]. The physiological effect of progesterone is as below[1]: during woman's normal menstrual cycle, an egg matures and is released from the ovaries[ovulation]. The ovary then produces progesterone to prevent the release of further eggs and priming the lining of the womb for a possible pregnancy. If pregnancy occurs, progesterone levels in the body remain high, maintaining the womb lining. If pregnancy does not occur, progesterone levels in the body fall, resulting in a menstrual period. Levonorgestrel tricks the body processes into thinking that ovulation has already occurred, by maintaining high levels of the synthetic progesterone. This prevents the release of eggs from the ovaries. |
|
Adverse reactions |
Side effects are medically minor but troublesome to the patients. No deaths or serious complications have been causally linked to LNG. Nausea and vomiting: The main side effects are gastrointestinal. The Yuzpe regimen causes nausea in up to 50% of recipients and as many as 19% experience vomiting. Since the pills are completely absorbed within one hour, replacement dosing is unnecessary if vomiting occurs after this time. The LNG regimen is significantly better tolerated, with nausea in 23% and vomiting 6%[14]. Meclizine is effective for preventing nausea and vomiting associated with the Yuzpe regimen of LNG[15]. Other common side effects with LNG regimens are dizziness, fatigue, headache, breast tenderness, lower abdominal pain and menstrual irregularities[14]. Although some women may experience spotting, the majority of women have their menstrual period on time or slightly early[16]. Risk of Ectopic pregnancy: Camp S.L. et al. has calculated from various clinical trials of 0.75 mg tablets of LNG used for EC(including almost 6000 women), 98 pregnancies were reported; 1 of which was ectopic, giving an ectopic pregnancy rate of 1%[9], i.e. we cannot deny a risk of ectopic pregnancy. Teratogenic Effects: The limited data on teratogenic effects come from a relatively small number of reports in which treatment was not successful, and the woman elected to continue the pregnancy. No evidence exists of a specific syndrome of anomalies or an apparent increase in the incidence of anomalies. It is important to recognize that no studies have investigated teratogenic effects associated with the use of oral emergency contraception[17]. |
|
Precautions/contraindication |
Women do not need provider intervention to use the LNG regimen of ECPs safely and effectively[18]. Progestogens only can be started in healthy non-pregnant women without screening procedures. According to WHO international guidelines, the minimum requirements before starting contraception with a combined estrogen -progestogens product consists of asking for a personal and family history of deep vein thrombosis and measuring blood pressure at baseline and follow-up. Combined agents are best avoided by women over 35 years who smoke[19]. If normal menstrual bleeding does not occur within 21 days after taking LNG or by 28 days if an oral contraceptive was initiated after LNG use, a pregnancy test should be taken[20]. Levonorgestrel is safe—even if taken in pregnancy, evidence suggests that it will not harm the pregnancy or the fetus —and it has no potential for addiction. The regimen has few contraindications, all of which should be easy for the consumer to identify for herself without needing an examination[21]. There are only three contraindications to the use of LNG as EC: existing pregnancy, undiagnosed vaginal bleeding or a known allergy to any ingredient in the product[9]. There are no absolute medical contraindications to the use of emergency contraception with the exception of pregnancy, and this is only because it is ineffective. According to WHO there are no known medical contraindications to the use of hormonal EC, aside from allergy to one of the constituents[22]. |
|
Chemical Properties |
White Solid |
|
Originator |
Ovrette,Wyeth,US,1968 |
|
Uses |
An emergency contraceptive. Levonorgestrel is safe, tolerated and effective in emergency contraception in woman |
|
Definition |
A synthetic steroid hormone used as an ingredient of oral contraceptives. Approved by FDA. |
|
Manufacturing Process |
To 0.7 gram of (+/-)-1,4-dihydro-17α-ethynyl-18-homo-oestradiol 3-methyl ether in 36 cc methanol was added 1.6 cc water and 2.4 cc concentrated hydrochloric acid. After standing at room temperature for 2 hours ether was added, and the washed and dried ethereal solution was evaporated, yielding a gum which was dissolved in 5 cc benzene and the solution absorbed on 50 grams of an activated fuller's earth. Elution with light petroleum containing increasing proportions of benzene gave a crystalline by-product: further elution with benzene containing a small proportion of ether gave a crystalline product which was recrystallized from ethyl acetate, yielding 0.11 gram of (+/-)-17α-ethynyl-18-homo-19-nortestosterone. MP 203° to 206°C. |
|
Brand name |
Mirena (Berlex); Norplant (Wyeth); Plan B (Duramed). |
|
Therapeutic Function |
Progestin |
|
Safety Profile |
Human reproductive effects byingestion: menstrual cycle changes, fertility index.Questionable carcinogen with experimental neoplastigenicdata. When heated to decomposition it emits acrid smokeand irritating fumes. |
InChI:InChI=1/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-19,23H,3,5-12H2,1H3/t16?,17?,18?,19-,20?,21-/m0/s1
797-63-7 Relevant articles
An improved alternative norgestrel preparation
Ferri?o, Sergio,López-Tapia, Francisco,Salgado-Zamora, Héctor
, p. 1461 - 1465 (1996)
Ethynyl cerium chloride, prepared in sit...
Synthesis method of levonorgestrel
-
Paragraph 0024; 0027, (2020/09/12)
The invention discloses a synthesis meth...
Preparation method of levonorgestrel
-
Paragraph 0025; 0027-0028; 0030-0031; 0033, (2020/09/20)
The invention discloses a preparation me...
Steroidal Molecular Rotors with 1,4-Diethynylphenylene Rotators: Experimental and Theoretical Investigations Toward Seeking Efficient Properties
?apiński, Andrzej,Farfán, Norberto,Górecki, Marcin,Jastrzebska, Izabella,Olszewska, Karolina,Runka, Tomasz,Santillan, Rosa
, p. 9625 - 9635 (2020/11/18)
Properly designed molecular rotors with ...
PROCESS FOR PREPARATION OF LEVONORGESTREL
-
Paragraph 0048, (2014/01/07)
The present invention provides an improv...
797-63-7 Process route
-
-
2-Ethyl-3-vinyl-cyclopentanone

-
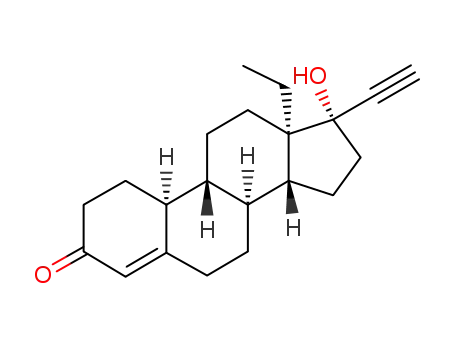
- 797-63-7,797-64-8,17092-09-0,6533-00-2
norgestrel
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 6 steps
1: 80 percent NaH, Et3N / 1,2-dimethoxy-ethane; paraffin / 1.) 3.5 h, reflux; 2.) 40 min
2: 1.) 2,4,6-trimethylphenol, pyridine; 2.) oxalic acid / 1.) methylcyclohexane, 13.5 h, 98 deg C, irradiation
3: 99 percent / LiAlH4 / diethyl ether
4: 62 percent / K, aniline, NH3 / tetrahydrofuran / 1 h
5: 76 percent / 2-butanone, aluminium isopropylate, mol. sieve / benzene
6: 0.16 g / LDA / tetrahydrofuran
With pyridine; lithium aluminium tetrahydride; molecular sieve; Mesitol; ammonia; oxalic acid; aluminum isopropoxide; sodium hydride; triethylamine; aniline; butanone; lithium diisopropyl amide; In tetrahydrofuran; 1,2-dimethoxyethane; diethyl ether; paraffin; benzene;
|
|
|
Multi-step reaction with 7 steps
1: 86 percent / TsOH*H2O / 14 h / Heating
2: 1.) 1.3 M MeLi; 2.) 10 percent H2SO4 / 1.) Et2O, 0.5 h; -20 deg C, 3h; 2.) Et2O, 14 h, RT
3: 1.) 2,4,6-trimethylphenol, pyridine; 2.) oxalic acid / 1.) methylcyclohexane, 13.5 h, 98 deg C, irradiation
4: 99 percent / LiAlH4 / diethyl ether
5: 62 percent / K, aniline, NH3 / tetrahydrofuran / 1 h
6: 76 percent / 2-butanone, aluminium isopropylate, mol. sieve / benzene
7: 0.16 g / LDA / tetrahydrofuran
With pyridine; lithium aluminium tetrahydride; molecular sieve; Mesitol; sulfuric acid; ammonia; methyllithium; oxalic acid; aluminum isopropoxide; toluene-4-sulfonic acid; aniline; butanone; lithium diisopropyl amide; In tetrahydrofuran; diethyl ether; benzene;
|
|
|
Multi-step reaction with 6 steps
1: 1.) NaH, 2.) H2SO4 / 1.) DME, paraffin oil; Et2O; 2.) MeOH
2: 1.) 2,4,6-trimethylphenol, pyridine; 2.) oxalic acid / 1.) methylcyclohexane, 13.5 h, 98 deg C, irradiation
3: 99 percent / LiAlH4 / diethyl ether
4: 62 percent / K, aniline, NH3 / tetrahydrofuran / 1 h
5: 76 percent / 2-butanone, aluminium isopropylate, mol. sieve / benzene
6: 0.16 g / LDA / tetrahydrofuran
With pyridine; lithium aluminium tetrahydride; molecular sieve; Mesitol; sulfuric acid; ammonia; oxalic acid; aluminum isopropoxide; sodium hydride; aniline; butanone; lithium diisopropyl amide; In tetrahydrofuran; diethyl ether; benzene;
|
-
![(5R,8R,9S,10R,13S,14S)-13-Ethyl-5-hydroxy-3-methyl-tetradecahydro-4-oxa-cyclopenta[a]phenanthren-17-one](/upload/2023/9/bbad4fc0-80fb-45f5-babe-5ef91e4981df.png)
- 23855-42-7,35442-18-3,35442-19-4
(5R,8R,9S,10R,13S,14S)-13-Ethyl-5-hydroxy-3-methyl-tetradecahydro-4-oxa-cyclopenta[a]phenanthren-17-one

-

- 797-63-7,797-64-8,17092-09-0,6533-00-2
norgestrel
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: NBS / acetone
2: (i) liq. NH3 (ii) /BRN= 4147360/, benzene, Et2O
With N-Bromosuccinimide; In acetone;
|
|
|
Multi-step reaction with 3 steps
1: CrO3, H2SO4 / acetone
2: TsOH / benzene
3: (i) liq. NH3 (ii) /BRN= 4147360/, benzene, Et2O
With chromium(VI) oxide; sulfuric acid; toluene-4-sulfonic acid; In acetone; benzene;
|
797-63-7 Upstream products
-
799-43-9
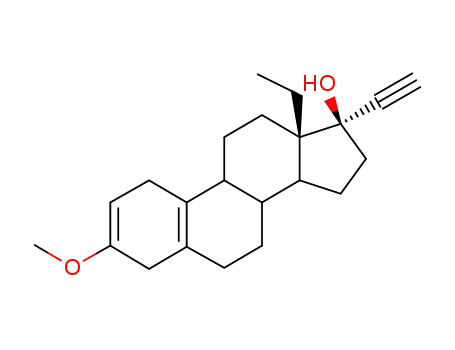
13β-ethyl-3-methoxy-17α-ethynyl-gona-2,5(10)-dien-17β-ol
-
21800-83-9
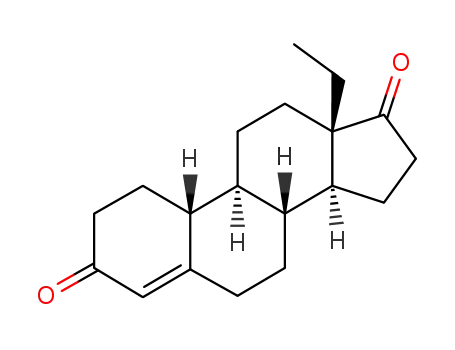
(+/-)-13β-Ethylgon-4-ene-3,17-dione
-
74-86-2
acetylene
-
2322-77-2
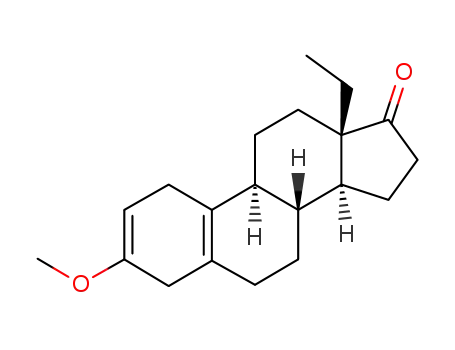
dl-3-methoxy-13β-ethylgona-2,5(10)-dien-17-one
797-63-7 Downstream products
-
797-58-0
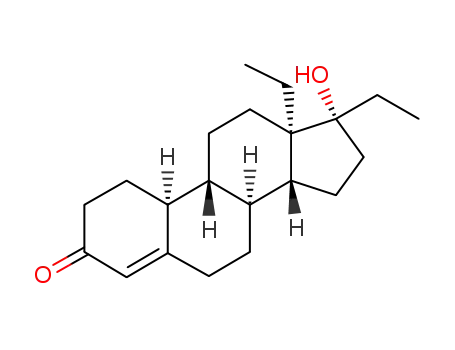
DL-17β-Hydroxy-13β.17α-diethyl-gon-4-en-3-on
-
13732-69-9
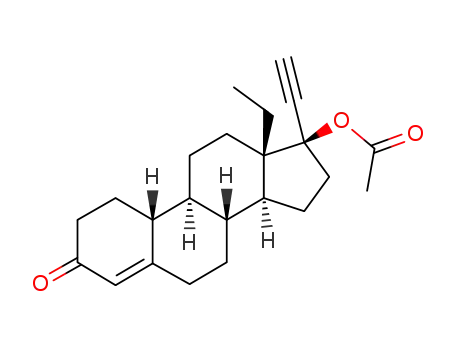
D-17β-acetoxy-13β-ethyl-17α-ethynyl-gon-4-en-3-one
-
78088-19-4
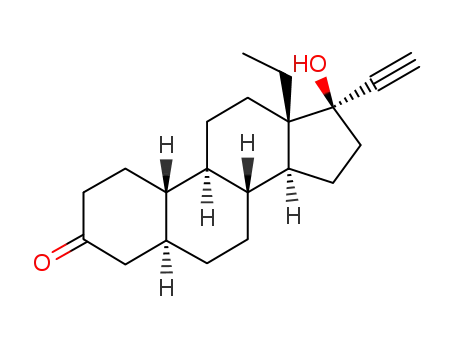
5alpha-LNG
-
100021-07-6
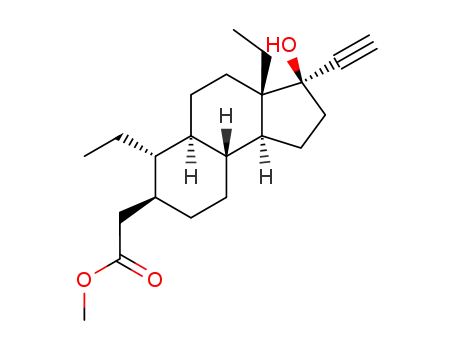
((3R,3aS,5aR,6S,7S,9aR,9bS)-3a,6-Diethyl-3-ethynyl-3-hydroxy-dodecahydro-cyclopenta[a]naphthalen-7-yl)-acetic acid methyl ester
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
TRANS-4-ETHYLCYCLOHEXANOL
CAS:19781-62-5