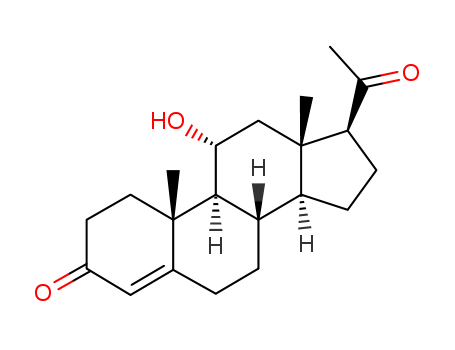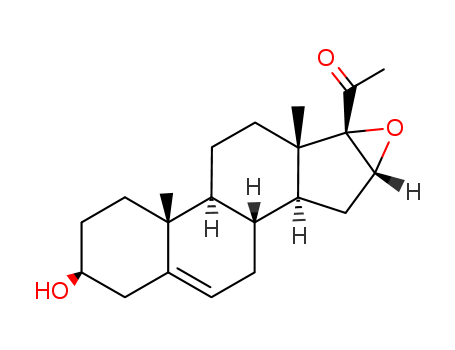
974-23-2
- Product Name:16,17a-Epoxy Pregnenolone
- Molecular Formula:C21H30O3
- Purity:99%
- Molecular Weight:330.467
Product Details;
CasNo: 974-23-2
Molecular Formula: C21H30O3
Factory Supply High Purity 16,17a-Epoxy Pregnenolone 974-23-2 Buy with Best Price
- Molecular Formula:C21H30O3
- Molecular Weight:330.467
- Vapor Pressure:1.73E-10mmHg at 25°C
- Melting Point:185-188 °C
- Refractive Index:1.577
- Boiling Point:462.3 °C at 760 mmHg
- PKA:14.99±0.70(Predicted)
- Flash Point:159.4 °C
- PSA:49.83000
- Density:1.19 g/cm3
- LogP:3.64660
16,17-Epoxypregnenolone(Cas 974-23-2) Usage
16,17-Epoxypregnenolone is an epoxy steroid that is pregnenolone with an epoxy group.
InChI:InChI=1/C21H30O3/c1-12(22)21-18(24-21)11-17-15-5-4-13-10-14(23)6-8-19(13,2)16(15)7-9-20(17,21)3/h4,14-18,23H,5-11H2,1-3H3
974-23-2 Relevant articles
Synthesis and activity of novel 16-dehydropregnenolone acetate derivatives as inhibitors of type 1 5α-reductase and on cancer cell line SK-LU-1
Silva-Ortiz, Aylin Viviana,Bratoeff, Eugene,Ramírez-Apan, Teresa,Heuze, Yvonne,Sánchez, Araceli,Soriano, Juan,Cabeza, Marisa
, p. 7535 - 7542 (2015)
Testosterone (T) plays a crucial role in...
The first syntheses of 16β-chloro- and 16β-bromo-cyproterone acetate
Sakee, Uthai,Kongkathip, Ngampong,Kongkathip, Boonsong
, p. 1695 - 1706 (2003)
The first syntheses of 16β-chloro- and 1...
Synthesis of new derivatives of 21-imidazolyl-16-dehydropregnenolone as inhibitors of 5α-reductase 2 and with cytotoxic activity in cancer cells
Silva-Ortiz, Aylin Viviana,Bratoeff, Eugene,Ramírez-Apan, Teresa,Heuze, Yvonne,Soriano, Juan,Moreno, Isabel,Bravo, Marisol,Bautista, Lucero,Cabeza, Marisa
, p. 1600 - 1607 (2017)
The aim of this study was to synthesize ...
Synthesis and biological activity of two pregnane derivatives with a triazole or imidazole ring at C-21
Silva-Ortiz, Aylin Viviana,Bratoeff, Eugene,Ramírez-Apan, María Teresa,García-Becerra, Rocío,Ordaz-Rosado, David,Noyola-Martínez, Nancy,Castillo-Bocanegra, Rafael,Barrera, David
, p. 8 - 18 (2016)
Pregnane derivatives are studied as agen...
Synthesis and transformations of 20-isoxazolylsteroids with modified D ring: I. Synthesis of 16α,17α-epoxyderivatives
Litvinovskaya,Drach,Khripach
, p. 787 - 792 (2001)
Synthesis of 16α,17α-epoxy-20-isoxazolyl...
Practical synthesis of 16α-bromo-17α-hydroxysteroids via a Raney Ni-catalyzed bromide exchange reaction
Xu, Fei-Fei,Li, Hong-Ping,Wang, Mao-Chang,Ma, Hai-Yan,Zhao, Mei-Xin,Ding, Kai
supporting information, p. 1710 - 1714 (2019/06/05)
D-ring modified glucocorticoids are attr...
Synthesis and cytotoxic effect of pregnenolone derivatives with one or two α,β-unsaturated carbonyls and an ester moiety at C-21 or C-3
Chávez-Riveros, Alejandra,Cruz Noriega, Abigail,Ramírez Apan, María Teresa,Miranda, Luis D.,Bratoeff, Eugene
, p. 37 - 45 (2018/02/06)
Four series of pregnenolone derivatives ...
Synthetic method for drug intermediate 5-pregnene-16alpha,17alpha-epoxy-3beta-ol-20-one
-
Paragraph 0012; 0014-0025, (2018/07/30)
The invention discloses a synthetic meth...
974-23-2 Process route
-
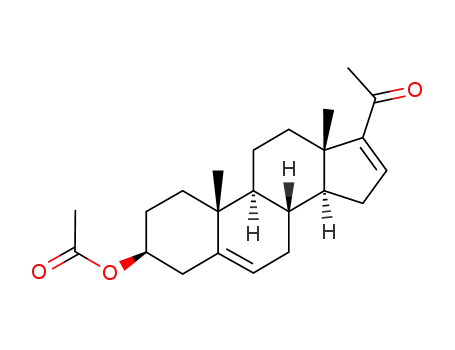
- 979-02-2
16-dehydropregnenolone acetate

-
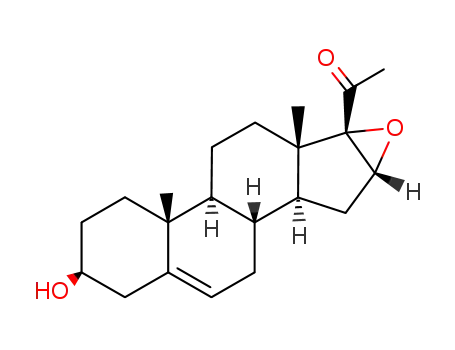
- 974-23-2
3β-hydroxy-16α,17α-epoxypregn-5-en-20-one
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; urea hydrogen peroxide adduct; In methanol; water; at 5 ℃; for 72h;
|
97% |
|
With sodium hydroxide; urea hydrogen peroxide adduct; In methanol; water; at 5 - 500 ℃; for 72h;
|
97% |
|
With sodium hydroxide; dihydrogen peroxide; In methanol; water; at 5 - 15 ℃; for 23h;
|
95% |
|
With dihydrogen peroxide; sodium hydroxide; In methanol; water; at 20 ℃; for 4h;
|
95% |
|
With sodium hydroxide; dihydrogen peroxide; In ethanol; at 30 - 40 ℃; for 2h;
|
91% |
|
With sodium hydroxide; dihydrogen peroxide; In methanol; water; at 5 ℃; for 24h;
|
89% |
|
With water; dihydrogen peroxide; sodium hydroxide; In methanol; at 20 ℃; for 4h;
|
72% |
|
With dihydrogen peroxide; sodium hydroxide; In methanol; at 20 ℃; for 4h;
|
72% |
|
With methanol; sodium hydroxide; dihydrogen peroxide;
|
|
|
With sodium hydroxide; dihydrogen peroxide; In methanol;
|
|
|
With hydroxide; dihydrogen peroxide; for 3.5h; Ambient temperature;
|
|
|
With hydroxide; dihydrogen peroxide; for 12h; Ambient temperature;
|
|
|
With sodium hydroxide; dihydrogen peroxide; at 20 ℃; for 12h;
|
|
|
With dihydrogen peroxide; sodium hydroxide;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydroxide / methanol
2: sodium hydroxide; dihydrogen peroxide / methanol / 4 h / 20 °C
With dihydrogen peroxide; sodium hydroxide; In methanol;
|
|
|
With dihydrogen peroxide; sodium hydroxide;
|
|
|
With dihydrogen peroxide; sodium hydroxide; In methanol; at 20 ℃; for 2.5h;
|
-
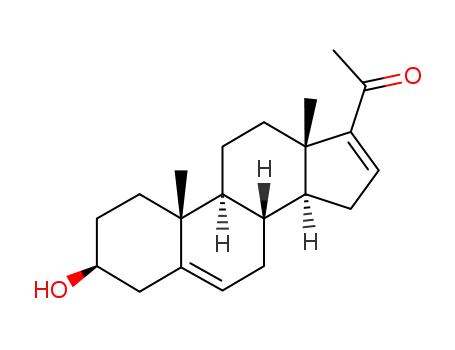
- 1162-53-4
16-dehydropregnenolone

-

- 974-23-2
3β-hydroxy-16α,17α-epoxypregn-5-en-20-one
| Conditions | Yield |
|---|---|
|
With tert.-butylhydroperoxide; lithium hydroxide; In ethanol; for 48h; Ambient temperature;
|
95% |
|
With dihydrogen peroxide; sodium hydroxide; In methanol;
|
|
|
With dihydrogen peroxide; sodium hydroxide; In methanol; at 20 ℃; for 4h;
|
0.87 mg |
974-23-2 Upstream products
-
979-02-2

16-dehydropregnenolone acetate
-
93-59-4
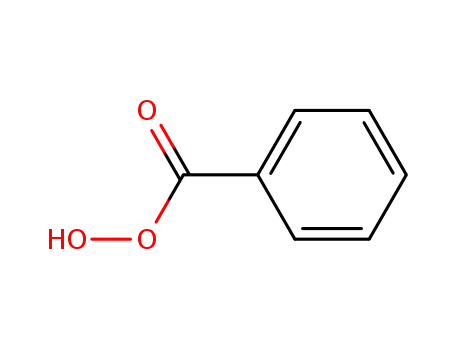
Perbenzoic acid
-
78792-99-1
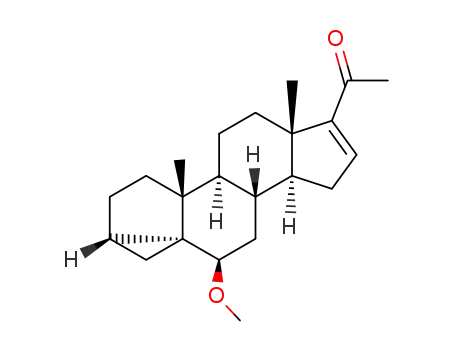
6β-methoxy-3α,5α-cyclo-pregn-16-en-20-one
-
911442-53-0
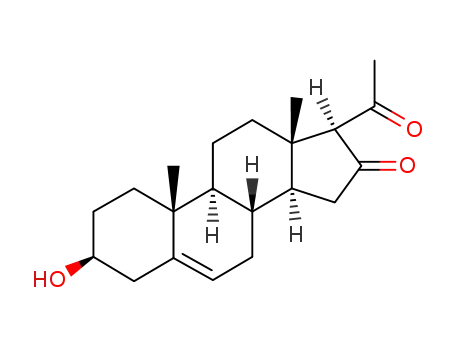
3β-hydroxypregn-5-ene-16,20-dione
974-23-2 Downstream products
-
34209-81-9
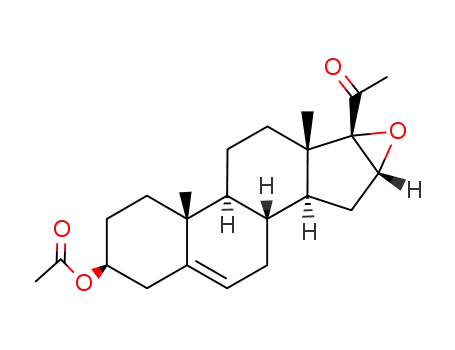
Acetic acid (3S,8R,9S,10R,13S,14S,16R,17S)-17-acetyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-20-oxa-cyclopropa[16,17]cyclopenta[a]phenanthren-3-yl ester
-
23357-24-6
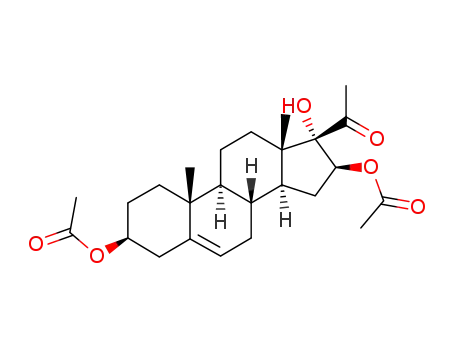
3β,16β-diacetoxy-17α-hydroxypregn-5-en-20-one
-
42995-32-4
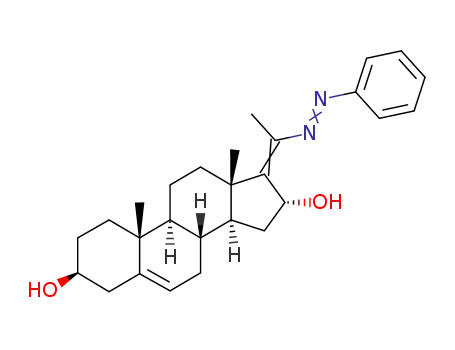
(20Ξ)-20-phenylazo-pregna-5,17(20)-diene-3β,16α-diol
-
26632-78-0
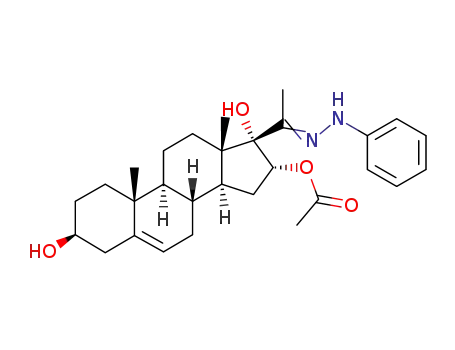
16α-acetoxy-3β,17-dihydroxy-pregn-5-en-20-one-phenylhydrazone
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
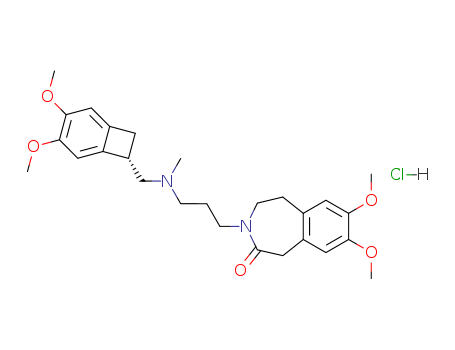
Ivabradine Hydrochloride
CAS:148849-67-6
-
11Α-Hydroxyprogesterone
CAS:80-75-1