1078-19-9
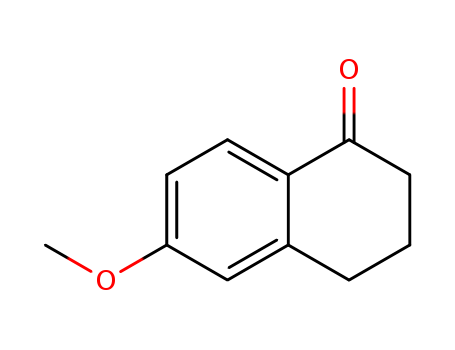
- Product Name:6-Methoxy-1-Tetralone
- Molecular Formula:C11H12O2
- Purity:99%
- Molecular Weight:176.215
Product Details;
CasNo: 1078-19-9
Molecular Formula: C11H12O2
Appearance: yellow crystalline powder
Quality Factory Supply Top Purity 6-Methoxy-1-Tetralone 1078-19-9 Lowest Price
- Molecular Formula:C11H12O2
- Molecular Weight:176.215
- Appearance/Colour:yellow crystalline powder
- Vapor Pressure:0.000528mmHg at 25°C
- Melting Point:77-79 ºC
- Refractive Index:1.548
- Boiling Point:312.5 ºC at 760 mmHg
- Flash Point:153.4 ºC
- PSA:26.30000
- Density:1.124 g/cm3
- LogP:2.21420
6-Methoxytetralone(Cas 1078-19-9) Usage
|
Chemical Properties |
yellow to light brown fine crystalline powder |
|
Uses |
6-Methoxy-1-tetralone is a reagent useful in the synthesis of (2-(furanyl)vinyl)-1-tetralone chalcones which possesses anticancer agents and inducers of apoptosis. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 64, p. 94, 1942 DOI: 10.1021/ja01253a025Tetrahedron Letters, 9, p. 2917, 1968 DOI: 10.1016/S0040-4039(00)89611-2 |
|
Synthesis |
The synthesis of?6-methoxy-1-tetralone is as follows:Eaton's reagent (1.00 mL, 5.31 mmol) was added slowly to a stirred solution of methyl 4-(3-methoxyphenyl)butanoate (4; 0.37g, 1.78 mmol) in DCE (1 mL). The resulting mixture was stirred at 75 °C for 2h under N2 atmosphere. Then, the reaction mixture was allowed to reach room temperature, and was poured over ice-water and extracted with EtOAc (3 × 15 mL). The combined organic extracts were washed successively with brine (2 × 15 mL) and H2O (2 × 20 mL), filtered over Na2SO4 and concentrated under reduced pressure. The resulting brownish oil was fractionated by column chromatography (silica gel, EtOAc-hexanes, 1:9) to obtain 6-methoxy-1-tetralone as ayellowish oil; yield: 0.28 g (91%). |
InChI:InChI=1/C11H12O2/c1-13-9-5-6-10-8(7-9)3-2-4-11(10)12/h5-7H,2-4H2,1H3
1078-19-9 Relevant articles
A facile and regioselective synthesis of 1-tetralones via silver-catalyzed ring expansion
Yu, Jiajia,Zhao, Huijun,Liang, Shuguang,Bao, Xiaoguang,Zhu, Chen
, p. 7924 - 7927 (2015)
A regioselective synthesis of 1-tetralon...
1,3-BENZODITHIOLIUM ION MEDIATED ANNULATIONS
Rigby, James H.,Kotnis, Atul,Kramer, James
, p. 2939 - 2940 (1983)
A mild and selective method for cyclizat...
Benzylic Oxidation Using tert-Butyl Hydroperoxide in the Presence of chromium Hexacarbonyl
Pearson, Anthony J.,Han, Gi Rin
, p. 2791 - 2792 (1985)
-
AN IMPROVED PREPARATION OF VINYL IODIDES
Barton, Derek H. R.,Bashiardes, George,Fourrey, Jean-Louis
, p. 1605 - 1608 (1983)
The oxidation of ketone hydrazones by io...
Intermolecular Oxidative Radical Addition to Aromatic Aldehydes: Direct Access to 1,4- and 1,5-Diketones via Silver-Catalyzed Ring-Opening Acylation of Cyclopropanols and Cyclobutanols
Che, Chao,Qian, Zhaosheng,Wu, Mingchang,Zhao, Ying,Zhu, Gangguo
, p. 5665 - 5673 (2018)
A novel silver-catalyzed ring-opening ac...
Chemoselective oxidation of benzylic alcohols with solid supported CrO3/TBHP under microwave irradiation
Singh,Sharma,Chhibber,Kaur,Kad
, p. 3941 - 3945 (2000)
The efficient use of microwave energy co...
An Expeditious Synthesis of 8-Methoxy-1-tetralone
Castillo-Rangel, Norma,Pérez-Díaz, José Oscar H.,Vázquez, Alfredo
, p. 2050 - 2056 (2016)
8-Methoxy-1-tetralone was synthesized in...
A new method for the conversion of enol phenyl thioethers to ketones
Grieco, Paul A.,Dai, Yujia
, p. 6997 - 7000 (1998)
Exposure of enol thioethers at ambient t...
PRACTICAL CHROMIUMVI OXIDE-CATALYZED BENZYLIC OXIDATIONS USING 70percent TERT-BUTYLHYDROPEROXIDE
Muzart, Jacques
, p. 2131 - 2132 (1987)
In using 70percent t.BuOOH and catalytic...
Selective electrochemical oxidation of aromatic hydrocarbons and preparation of mono/multi-carbonyl compounds
Li, Zhibin,Zhang, Yan,Li, Kuiliang,Zhou, Zhenghong,Zha, Zhenggen,Wang, Zhiyong
, p. 2134 - 2141 (2021/09/29)
A selective electrochemical oxidation wa...
Mechanical metal activation for Ni-catalyzed, Mn-mediated cross-electrophile coupling between aryl and alkyl bromides
Wu, Sisi,Shi, Weijia,Zou, Gang
supporting information, p. 11269 - 11274 (2021/07/02)
Liquid-assisted grinding has been succes...
Regioselective Electrochemical Cyclobutanol Ring Expansion to 1-Tetralones
Petti, Alessia,Natho, Philipp,Lam, Kevin,Parsons, Philip J.
, p. 854 - 858 (2021/01/12)
A mild electrochemical method for the re...
Ruthenium-Catalyzed Dehydrogenation of Alcohols with Carbodiimide via a Hydrogen Transfer Mechanism
Sueki, Shunsuke,Matsuyama, Mizuki,Watanabe, Azumi,Kanemaki, Arata,Katakawa, Kazuaki,Anada, Masahiro
, p. 4878 - 4885 (2020/06/02)
Ruthenium-catalyzed oxidative dehydrogen...
1078-19-9 Process route
-
- 24743-11-1
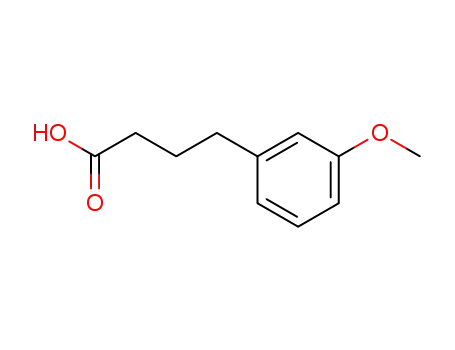
4-(m-methoxyphenyl)butanoic acid

-
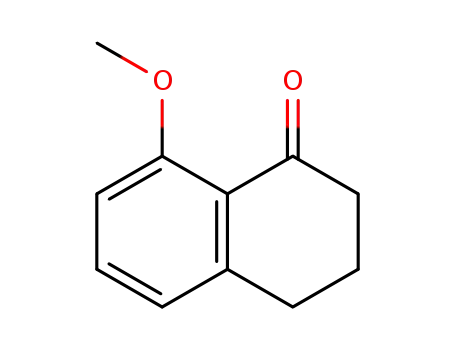
- 13185-18-7
8-methoxy-1-tetralone

-
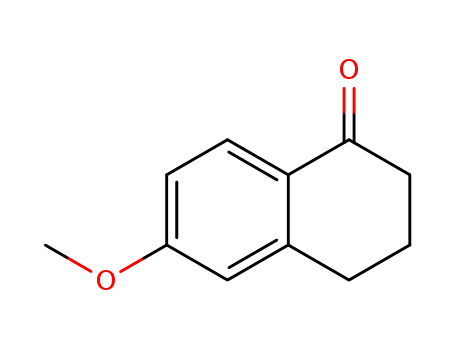
- 1078-19-9
6-methoxy-3,4-dihydro-1(2H)-naphthalenone
| Conditions | Yield |
|---|---|
|
With polyphosphoric ester; In chloroform; for 7h; Yield given. Yields of byproduct given; Ambient temperature;
|
-
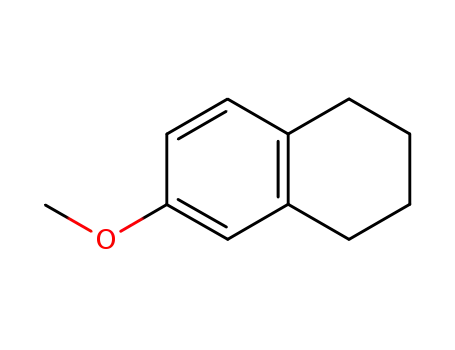
- 1730-48-9
6-methoxy-1,2,3,4-tetrahydronaphthalene

-
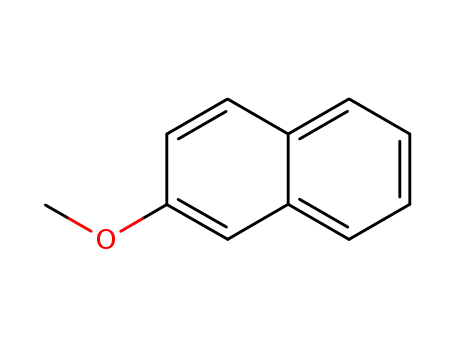
- 93-04-9
2-Methoxynaphthalene

-

- 1078-19-9
6-methoxy-3,4-dihydro-1(2H)-naphthalenone
| Conditions | Yield |
|---|---|
|
With dipotassium peroxodisulfate; copper(I) sulfate; In water; acetonitrile; at 65 - 70 ℃; for 3h;
|
10% 45% |
1078-19-9 Upstream products
-
104-90-5
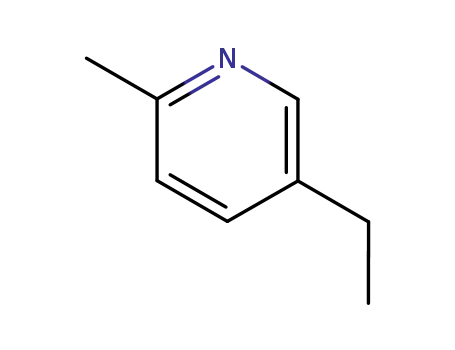
5-ethyl-2-methyl-pyridine
-
1730-48-9

6-methoxy-1,2,3,4-tetrahydronaphthalene
-
20710-24-1
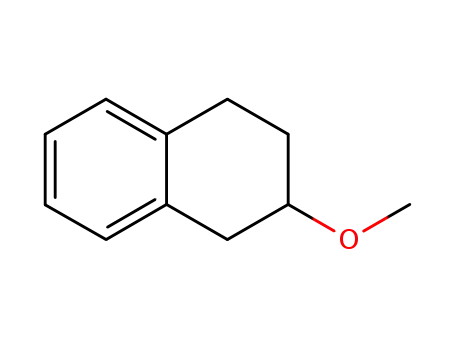
3-methoxytetralin
-
24743-11-1

4-(m-methoxyphenyl)butanoic acid
1078-19-9 Downstream products
-
495394-10-0
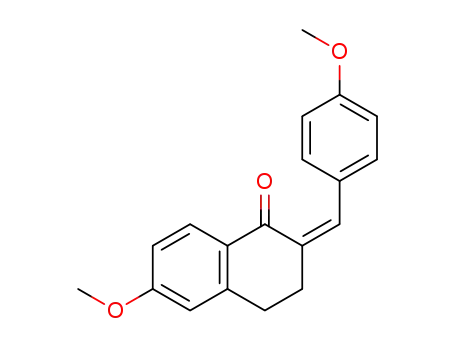
(paramethoxybenzylidene)-2 methoxy-6 tetralone-1
-
68254-80-8
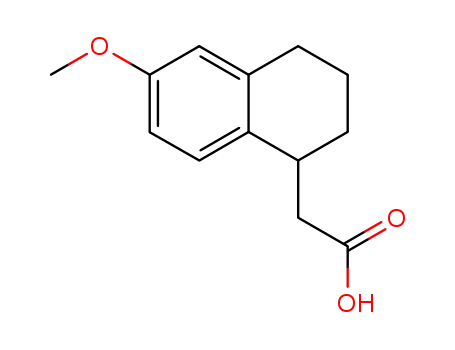
6'-methoxy-1',2',3',4'-tetrahydro-1'-naphthylacetic acid
-
72861-81-5
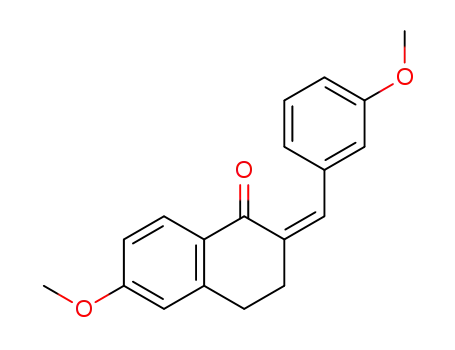
6-methoxy-2-((Z)-3-methoxy-benzylidene)-3,4-dihydro-2H-naphthalen-1-one
-
79822-57-4
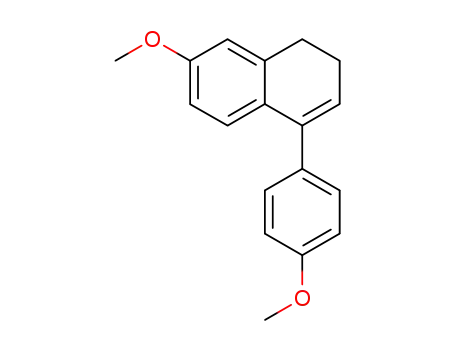
7-methoxy-4-(4-methoxyphenyl)-1,2-dihydronaphthalene
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
trans-(4-(trifluoromethyl)cyclohexyl)methanol
CAS:1202577-61-4
-
clindamycin phosphate
CAS:24729-96-2