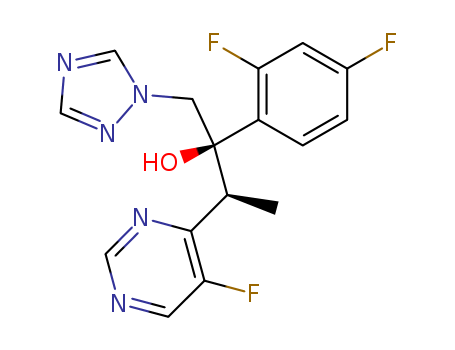
4897-25-0
- Product Name:1-Methyl-4-Nitro-5-Chloro Imidazole
- Molecular Formula:C4H4ClN3O2
- Purity:99%
- Molecular Weight:161.548
Product Details;
CasNo: 4897-25-0
Molecular Formula: C4H4ClN3O2
Appearance: White solid
Buy High Quality Reliable Quality 1-Methyl-4-Nitro-5-Chloro Imidazole 4897-25-0 Cheapest Price
- Molecular Formula:C4H4ClN3O2
- Molecular Weight:161.548
- Appearance/Colour:White solid
- Vapor Pressure:4.08E-05mmHg at 25°C
- Melting Point:148-150 °C(lit.)
- Refractive Index:1.648
- Boiling Point:362.3 °C at 760 mmHg
- PKA:-1.37±0.61(Predicted)
- Flash Point:172.9 °C
- PSA:63.64000
- Density:1.65 g/cm3
- LogP:1.50490
5-Chloro-1-methyl-4-nitroimidazole(Cas 4897-25-0) Usage
|
Description |
5-Chloro-1-methyl-4-nitroimidazole is an imidazole derivative. It is useful in the rapid mix experiments to investigate the mechanism of anomalous radiosensitization of mammalian cells. It can also be used in the synthesis of 5-aryl-1-methyl-4-nitroimidazoles, via Suzuki coupling with arylboronic acids, catalyzed by dichlorobis-(triphenylphosphine) palladium (II), K2CO3 and tetrabutylammonium bromide. |
|
Chemical Properties |
White Solid |
|
Uses |
5-Chloro-1-methyl-4-nitroimidazole (CMNI) is suitable for use in the rapid mix experiments to investigate the mechanism of anomalous radiosensitization of mammalian cells by CMNI. It may be used in the synthesis of 5-aryl-1-methyl-4-nitroimidazoles, via Suzuki coupling with arylboronic acids, catalyzed by dichlorobis-(triphenylphosphine)palladium(II), K2CO3 and tetrabutylammonium bromide. |
|
General Description |
5-Chloro-1-methyl-4-nitroimidazole is an 4-nitroimidazole derivative. |
|
Contact allergens |
This intermediate in azathioprine synthesis is also present in the end product. It induced contact dermatitis in a man working on azathioprine synthesis. Cross-reactivity is possible with imidazoles tioconazole and econazole. |
InChI:InChI=1/C4H4ClN3O2/c1-7-2-6-4(3(7)5)8(9)10/h2H,1H3
4897-25-0 Relevant articles
Rational design of novel immunosuppressive drugs: Analogues of azathioprine lacking the 6-mercaptopurine substituent retain or have enhanced immunosuppressive effects
Crawford, Duncan J. K.,Maddocks, John L.,Jones, D. Neville,Szawlowski, Paul
, p. 2690 - 2695 (1996)
Clinical use of the immunosuppressive dr...
4,5-DIAMINOIMIDAZOLES AS NOVEL DEVELOPER-TYPE OXIDATION DYE PRECURSORS
-
Paragraph 0277-0280, (2017/10/22)
An agent for oxidative changing of the c...
HETEROCYCLO-SUBSTITUTED IMIDAZOPYRAZINE PROTEIN TYROSINE KINASE INHIBITORS
-
Page/Page column 39-40, (2009/06/27)
Novel heterocyclo-substituted imidazopyr...
ortho-Substituted azoles as selective and dual inhibitors of VEGF receptors 1 and 2
Kiselyov, Alexander S.,Piatnitski, Evgueni L.,Samet, Alexander V.,Kisliy, Victor P.,Semenov, Victor V.
, p. 1369 - 1375 (2007/10/03)
We have developed a series of novel pote...
Hetaryl imidazoles: A novel dual inhibitors of VEGF receptors I and II
Kiselyov, Alexander S.,Semenova, Marina,Semenov, Victor V.
, p. 1440 - 1444 (2007/10/03)
A novel potent derivatives of hetaryl im...
4897-25-0 Process route
-
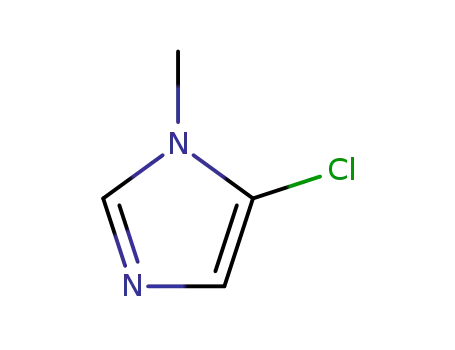
- 872-49-1
N-methyl-5-chloroimidazole

-
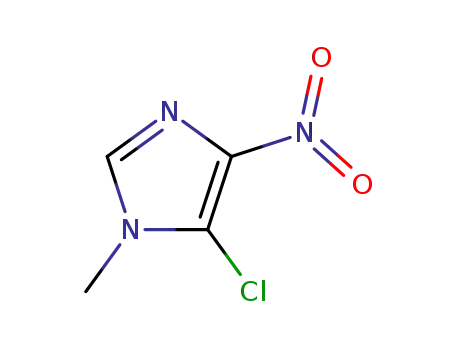
- 4897-25-0
5-chloro-1-methyl-4-nitroimidazole
| Conditions | Yield |
|---|---|
|
With sulfuric acid; nitric acid; In water; Heating;
|
91% |
|
With sulfuric acid; nitric acid; at 100 ℃; for 3.41667h; Inert atmosphere; Cooling with ice;
|
62% |
|
With sulfuric acid; nitric acid;
|
|
|
With sulfuric acid; nitric acid; at 90 ℃; for 2h;
|
|
|
With sulfuric acid; nitric acid; In water; at 95 ℃; for 2h;
|
-
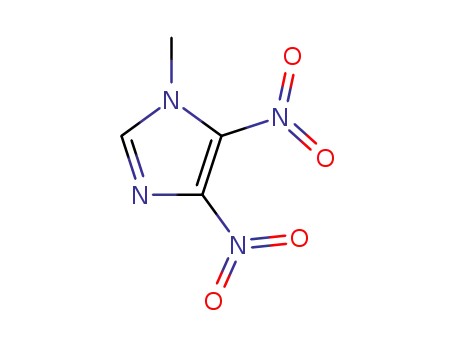
- 19183-15-4
1-methyl-4,5-dinitro-1H-imidazole

-

- 4897-25-0
5-chloro-1-methyl-4-nitroimidazole
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; for 3h; Heating;
|
74% |
4897-25-0 Upstream products
-
186581-53-3

diazomethane
-
57531-38-1
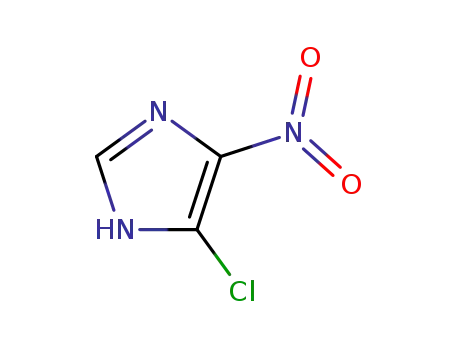
4-chloro-5-nitroimidazole
-
77-78-1
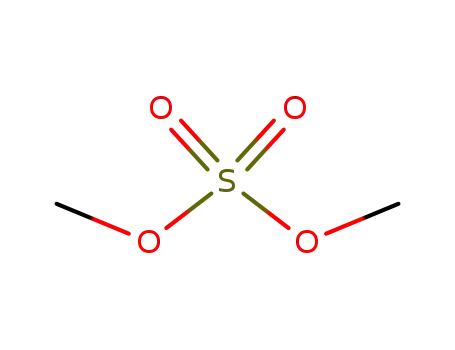
dimethyl sulfate
-
57531-38-1
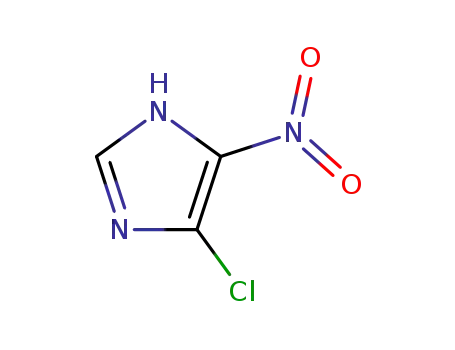
4(5)-Chloro-5(4)-nitroimidazol
4897-25-0 Downstream products
-
37527-30-3
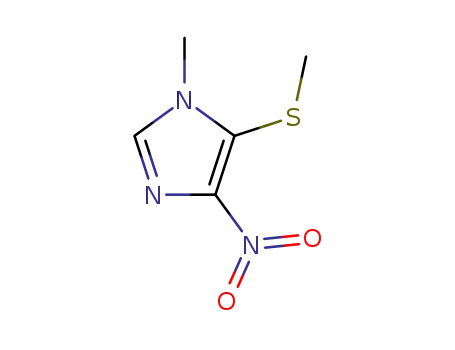
1-methyl-5-methylthio-4-nitroimidazole
-
5702-78-3
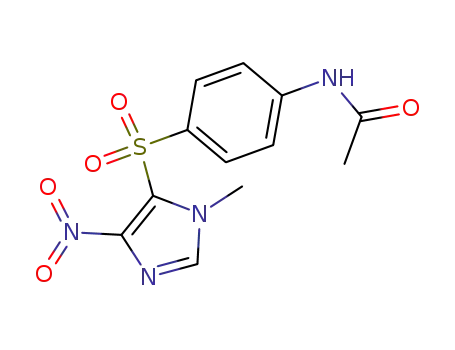
1-methyl-4-nitro-5-[4-acetamido(phenylsulfonyl)]-1H-imidazole
-
40648-96-2
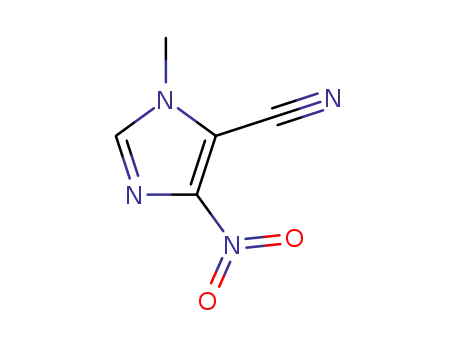
1-methyl-4-nitro-1H-imidazole-5-carbonitrile
-
124641-97-0
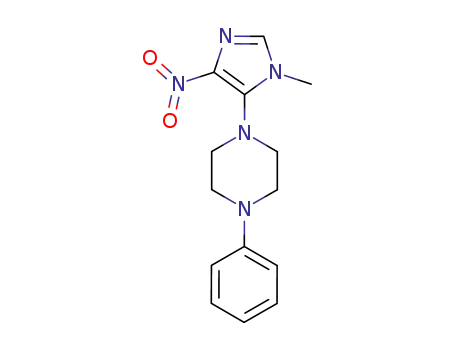
1-Methyl-4-nitro-5-(N-phenylpiperazino)-imidazole
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
Cyclohexanamine, 3-(trifluoromethyl)-
CAS:56287-83-3
-
Voriconazole
CAS:137234-62-9