
1162-56-7
- Product Name:6-dehydrogesterone
- Molecular Formula:C21H28 O2
- Purity:99%
- Molecular Weight:312.452
Product Details;
CasNo: 1162-56-7
Molecular Formula: C21H28 O2
Factory Sells Buy High Quality 6-dehydrogesterone 1162-56-7 Safe Shipping
- Molecular Formula:C21H28 O2
- Molecular Weight:312.452
- Vapor Pressure:9.55E-09mmHg at 25°C
- Melting Point:147-148 °C(Solv: hexane (110-54-3))
- Boiling Point:462.8 °C at 760 mmHg
- Flash Point:172.2 °C
- PSA:34.14000
- Density:1.1 g/cm3
- LogP:4.49950
Pregna-4,6-diene-3,20-dione(Cas 1162-56-7) Usage
|
Uses |
6-Dehydroprogesterone is an impurity found in progesterone. |
InChI:InChI=1/C21H28O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4-5,12,16-19H,6-11H2,1-3H3/t16-,17+,18-,19-,20-,21+/m0/s1
1162-56-7 Relevant articles
Synthesis of novel 7α-thiol-bridged progesterone derivatives
Wynne, James H.,Lloyd, Christopher T.,Mushrush, George W.
, p. 885 - 893 (2003)
Novel synthetic routes to the formation ...
-
Meystre,Wettstein
, p. 408 (1946)
-
Manganese mediated oxidation of progesterone in alkaline medium: Mechanism study and quantitative determination
Shamsipur, Mojtaba,Pashabadi, Afshin,Taherpour, Avat (Arman),Bahrami, Kiumars,Sharghi, Hashem
, p. 292 - 302 (2017)
We report here a non-immunosensing appro...
-
McAleer et al.
, p. 958 (1958)
-
-
Sondheimer et al.
, p. 5932,5934 (1953)
-
Synthesis of 21-diazoprogesterone-6,7-3H2
Chiu,Wolff
, p. 397 - 405 (1979)
The preparation of 21-diazoprogesterone-...
Method and compound for synthetizing reprogestin
-
Paragraph 0083-0088; 0089-0092; 0093-0096, (2021/09/15)
To the synthesis method, dehydropregneno...
Method for synthesizing dydrogesterone
-
Paragraph 0029-0031, (2021/05/19)
The invention relates to a method for sy...
Production process capable of industrially synthesizing dydrogesterone
-
Paragraph 0090-0094, (2020/03/03)
The invention discloses a production pro...
Preparation method for 5,7-pregnadien-3,20-dion-diethylene glycol ketal
-
, (2020/03/06)
The invention provides a preparation met...
1162-56-7 Process route
-
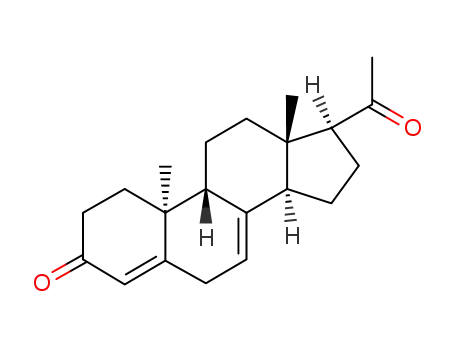
- 16484-70-1
7-Dehydro-9β,10α-progesteron

-
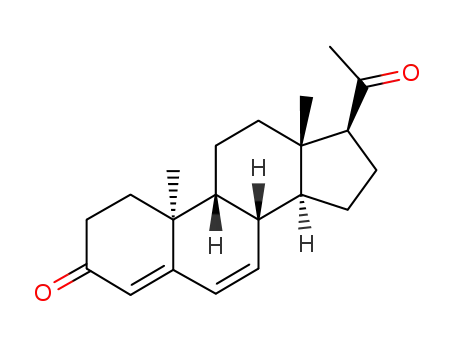
- 601-33-2,1162-56-7,2640-38-2,3795-24-2,25093-47-4,152-62-5
dydrogesterone
| Conditions | Yield |
|---|---|
|
With hydrogen bromide; acetic acid; at 0 ℃; Reagent/catalyst;
|
86.3% |
|
With hydrogenchloride; In tetrahydrofuran; water; at -5 ℃; Solvent; Temperature;
|
85% |
|
With hydrogenchloride; In water; isopropyl alcohol; at 10 ℃; for 2.5h; Reagent/catalyst; Solvent;
|
70% |
|
With hydrogenchloride; In isopropyl alcohol;
|
-
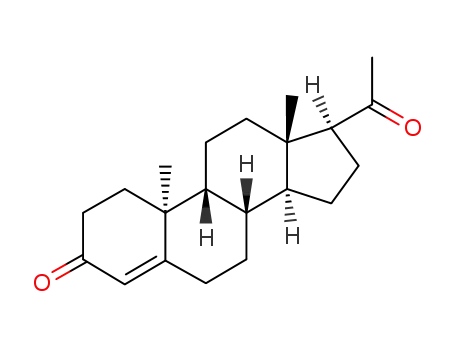
- 2755-10-4
(9β,10α)-pregn-4-ene-3,20-dione

-

- 601-33-2,1162-56-7,2640-38-2,3795-24-2,25093-47-4,152-62-5
dydrogesterone
| Conditions | Yield |
|---|---|
|
With chloranil; In tert-butyl alcohol; at 20 - 85 ℃; Reagent/catalyst; Temperature; Inert atmosphere;
|
1162-56-7 Upstream products
-
16484-70-1

7-Dehydro-9β,10α-progesteron
-
10072-88-5
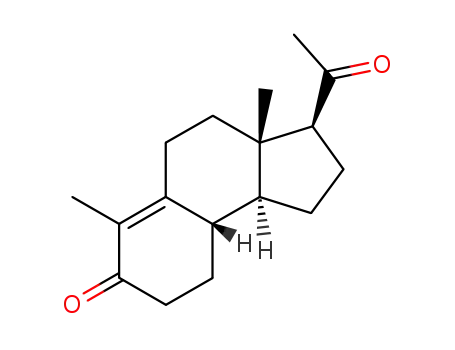
Des-A-17α-Δ9-pregnen-5,20-dion
-
2755-10-4

(9β,10α)-pregn-4-ene-3,20-dione
-
57-83-0
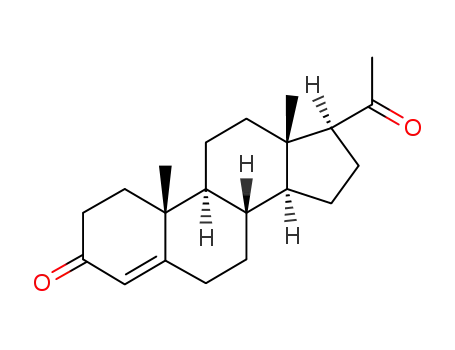
Progesterone
1162-56-7 Downstream products
-
21063-87-6
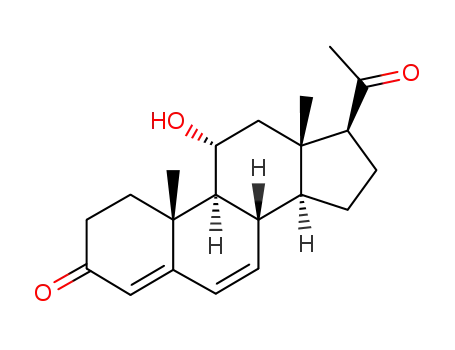
11α-hydroxypregna-4,6-diene-3,20-dione
-
57-83-0

Progesterone
-
600-99-7
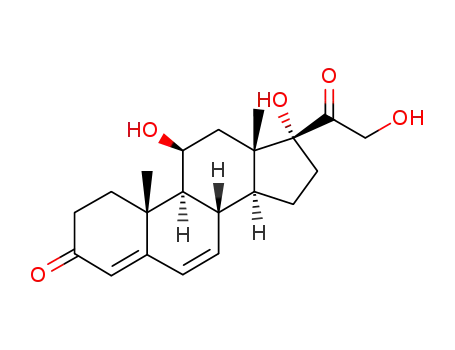
11β,17,21-trihydroxy-pregna-4,6-diene-3,20-dione
-
40845-01-0
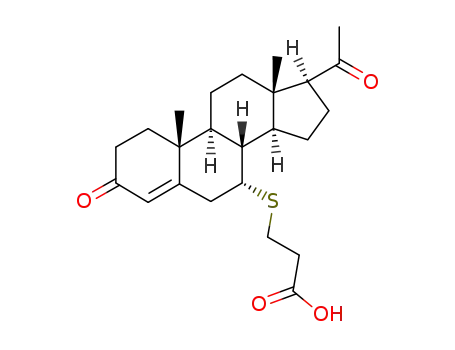
3-(pregn-4-ene-3,20-dione-7α-yl)thiopropanoic acid
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
2-Methoxy-6-(methylamino)pyridine
CAS:88569-83-9
-
3,6-Difluoropyrazine-2-carbonitrile
CAS:356783-28-3













