57149-07-2
- Product Name:Naftopidil
- Molecular Formula:C24H28N2O3
- Purity:99%
- Molecular Weight:392.498
Product Details;
CasNo: 57149-07-2
Molecular Formula: C24H28N2O3
Appearance: Off-White Solid
Chinese Manufacturer Supply Wholesale Naftopidil 57149-07-2 On Stock
- Molecular Formula:C24H28N2O3
- Molecular Weight:392.498
- Appearance/Colour:Off-White Solid
- Vapor Pressure:2.22E-15mmHg at 25°C
- Melting Point:127 °C
- Refractive Index:1.6300 (estimate)
- Boiling Point:602.8 °C at 760 mmHg
- PKA:14.01±0.20(Predicted)
- Flash Point:318.3 °C
- PSA:45.17000
- Density:1.184 g/cm3
- LogP:3.41320
Naftopidil dihydrochloride(Cas 57149-07-2) Usage
|
Description |
Naftopidil was launched in Japan for the treatment of dysuria associated with benign prostatic hypertrophy (BPH). It can be prepared by a two step route starting with α-naphthol. Naftopidil is a potent postsynaptic-selective alpha-l-antagonist with a slightly higher affinity for the human prostatic than for the aortic alpha-adrenoceptor. It also shows a 5-HT1A agonistic effect, as well as a weak calcium antagonistic activity, but no alpha-2 or beta-adrenoreceptor affinity. In experiments with rats or rabbits, Naftopidil was shown to be more potent and selective for the urodynamic effect than the hypotensive effect. Aromatic or aliphatic hydroxylation are the major routes of metabolism, producing metabolites with a profile similar to the parent compound. |
|
Chemical Properties |
Off-White Solid |
|
Originator |
Boehringer Mannheim (Germany) |
|
Uses |
Naftopidil is an α-1-adrenergic receptor antagonist. Naftopidil is used as an antihypertensive. |
|
Brand name |
Avishot;Flivas |
|
Biological Activity |
An α 1 -adrenoceptor antagonist with only weak antagonism at post-junctional α 2 receptors; a potent, persistent antihypertensive and vasodilator. |
InChI:InChI=1/C24H28N2O3.2ClH/c1-28-24-11-5-4-10-22(24)26-15-13-25(14-16-26)17-20(27)18-29-23-12-6-8-19-7-2-3-9-21(19)23;;/h2-12,20,27H,13-18H2,1H3;2*1H
57149-07-2 Relevant articles
NAFTOPIDIL MONOHYDROCHLORIDE DIHYDRATE AND USE THEREOF FOR PREPARATION OF NAFTOPIDIL
-
, (2021/07/27)
PROBLEM TO BE SOLVED: To provide an inte...
Continuous flow upgrading of glycerol toward oxiranes and active pharmaceutical ingredients thereof
Morodo, Romain,Gérardy, Romaric,Petit, Guillaume,Monbaliu, Jean-Christophe M.
, p. 4422 - 4433 (2019/08/21)
A robust continuous flow procedure for t...
New anticancer agent
-
Paragraph 0064-0066, (2017/04/03)
PROBLEM TO BE SOLVED: To provide a compo...
NOVEL ANTICANCER AGENT
-
Paragraph 0179-0181, (2015/12/30)
The present invention aims to provide a ...
57149-07-2 Process route
-

-
2461-42-9
3-(1-naphthyloxy)-1,2-epoxypropane

-

-
35386-24-4
1-(2-Methoxyphenyl)piperazine

-

-
57149-07-2
naftopidil
| Conditions | Yield |
|---|---|
|
In
ethanol;
for 1h;
Reflux;
|
100% |
|
In
ethanol;
at 20 ℃;
for 1h;
|
100% |
|
With
zinc(II) perchlorate hexahydrate;
In
dichloromethane;
at 20 ℃;
for 16h;
|
92% |
|
|
|
|
With
zinc(II) perchlorate;
In
water;
at 140 ℃;
for 0.166667h;
under 5250.53 Torr;
Flow reactor;
|
95 %Chromat. |
-
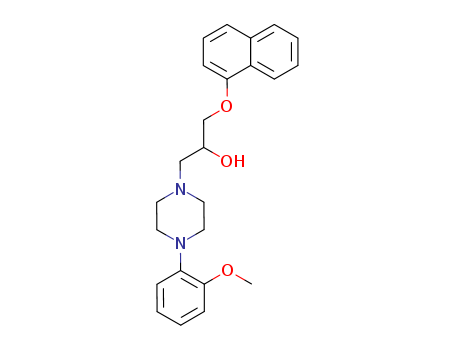
![(2RS)-1-[4-(2-methoxyphenyl)piperazin-1-yl]-3-(naphthalene-1-yloxy)propan-2-ol monohydrochloride](/upload/2023/9/f3947e3e-df67-4a13-a7e6-2feb786abf0b.png)
-
1164469-60-6,57149-08-3
(2RS)-1-[4-(2-methoxyphenyl)piperazin-1-yl]-3-(naphthalene-1-yloxy)propan-2-ol monohydrochloride

-

-
57149-07-2
naftopidil
| Conditions | Yield |
|---|---|
|
With
water; sodium hydroxide;
In
toluene;
Reagent/catalyst;
|
90% |
57149-07-2 Upstream products
-
2461-42-9

3-(1-naphthyloxy)-1,2-epoxypropane
-
35386-24-4

1-(2-Methoxyphenyl)piperazine
-
90-15-3

α-naphthol
-
90-04-0

2-methoxy-phenylamine
57149-07-2 Downstream products
-
133347-36-1

O-desmethyl-naftopidil
-
133024-36-9

(naphthyl)hydroxy-naftopidil
-
133024-35-8

(phenyl)hydroxy-naftopidil
-
117067-06-8

3-<4-(2-Methoxyphenyl)piperazinyl>-1,2-propandiol
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
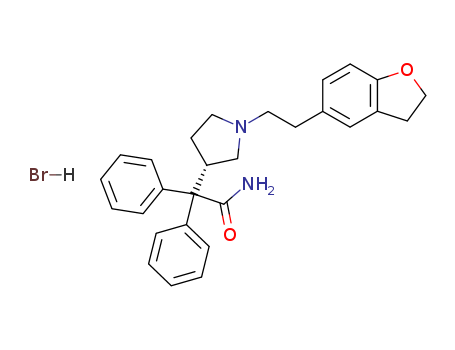
Darifenacin Hydrobromide
CAS:133099-07-7
-
1-(2-methoxyphenyl)Piperazine hydrobromide
CAS:100939-96-6