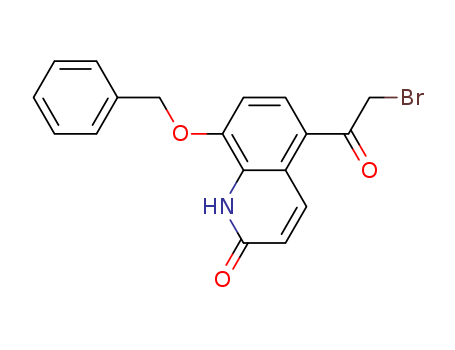
100331-89-3
- Product Name:8-benzyloxy-5-(2-bromoacetyl)-2-hydroxyquinoline
- Molecular Formula:C18H14BrNO3
- Purity:99%
- Molecular Weight:372.218
Product Details;
CasNo: 100331-89-3
Molecular Formula: C18H14BrNO3
Appearance: Off-white solid
Buy High Quality High Purity 8-benzyloxy-5-(2-bromoacetyl)-2-hydroxyquinoline 100331-89-3 Safe Transportation
- Molecular Formula:C18H14BrNO3
- Molecular Weight:372.218
- Appearance/Colour:Off-white solid
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.638
- Boiling Point:602.645 °C at 760 mmHg
- PKA:10.38±0.70(Predicted)
- Flash Point:318.268 °C
- PSA:59.42000
- Density:1.48 g/cm3
- LogP:4.09700
8-BENZYLOXY-5-(2-BROMOACETYL)-2-HYDROXYQUINOLINE(Cas 100331-89-3) Usage
|
Chemical Properties |
Off-white Solid |
|
Uses |
8-(benzyloxy)-5-(2-broMoacetyl)quinolin-2(1H)-one can be used in the preparation of phenylethanolamine derivatives as β2 adrenoreceptor agonists. |
InChI:InChI=1/C18H14BrNO3/c19-10-15(21)13-6-8-16(18-14(13)7-9-17(22)20-18)23-11-12-4-2-1-3-5-12/h1-9H,10-11H2,(H,20,22)
100331-89-3 Relevant articles
Discovery of a novel class of inhaled dual pharmacology muscarinic antagonist and β2 agonist (MABA) for the treatment of chronic obstructive pulmonary disease (COPD)
Rancati, Fabio,Linney, Ian D.,Rizzi, Andrea,Delcanale, Maurizio,Knight, Chris K.,Schmidt, Wolfgang,Pastore, Fiorella,Riccardi, Benedetta,Mileo, Valentina,Carnini, Chiara,Cesari, Nicola,Blackaby, Wesley P.,Patacchini, Riccardo,Carzaniga, Laura
supporting information, (2021/04/12)
The targeting of both the muscarinic and...
CLASS OF BIFUNCTIONAL COMPOUNDS WITH QUATERNARY AMMONIUM SALT STRUCTURE
-
Paragraph 0159, (2019/11/11)
The invention provides a class of compou...
Discovery of β-arrestin-biased β2-adrenoceptor agonists from 2-amino-2-phenylethanol derivatives
Woo, Anthony Yiu-Ho,Ge, Xin-yue,Pan, Li,Xing, Gang,Mo, Yong-mei,Xing, Rui-juan,Li, Xiao-ran,Zhang, Yu-yang,Wainer, Irving W.,Cheng, Mao-sheng,Xiao, Rui-ping
, p. 1095 - 1105 (2019/01/19)
β-Arrestins are a small family of protei...
A Process for Preparing Indacaterol and Salts Thereof
-
Paragraph 0070, (2018/08/20)
The present invention relates to a proce...
100331-89-3 Process route
-
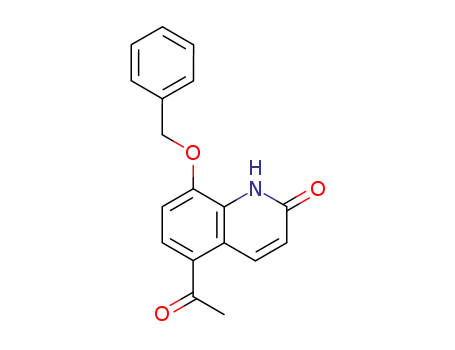
- 93609-84-8
5-acetyl-8-benzyloxy-1H-quinolin-2-one

-
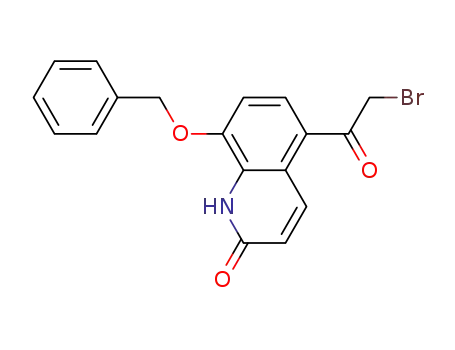
- 100331-89-3
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one
| Conditions | Yield |
|---|---|
|
With aluminum (III) chloride; bromine; In dichloromethane; at 0 - 20 ℃; for 4.5h;
|
82.9% |
|
With bromine; trifluoroborane diethyl ether; potassium carbonate; In dichloromethane;
|
75.7% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
With tetrabutylammomium bromide; In tetrahydrofuran; methanol; at 20 ℃;
|
73% |
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; In dichloromethane; at 0 - 20 ℃;
With bromine; In dichloromethane; at 45 ℃; for 0.916667h;
With sodium carbonate; In water; for 1h;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; In dichloromethane; at 0 - 20 ℃;
With bromine; In dichloromethane; at 45 ℃; for 0.916667h;
|
|
|
With bromine; trifluoroborane diethyl ether; In dichloromethane; at 0 - 45 ℃; for 0.25h;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With tetrabuthylammonium tribromide; In tetrahydrofuran; methanol; at 20 ℃; for 18.5h;
With water; In tetrahydrofuran; methanol; at 20 ℃; for 0.5h;
|
|
|
With boron trifluoride diethyl etherate; bromine; In dichloromethane; at 0 - 45 ℃; for 0.916667h;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; In dichloromethane; at 0 - 20 ℃;
With bromine; In dichloromethane; at 45 ℃; for 0.916667h;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; bromine; In dichloromethane; at 0 - 45 ℃; for 0.916667h;
With sodium carbonate; In water; for 1h;
|
|
|
With N-Bromosuccinimide; In chloroform;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; In dichloromethane; at 0 - 45 ℃;
With bromine; In dichloromethane; at 45 ℃; for 0.916667h;
|
|
|
With boron trifluoride diethyl etherate; bromine; In dichloromethane; at 0 - 45 ℃; for 0.916667h;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With trifluoroborane diethyl ether; In dichloromethane; at 0 - 20 ℃;
With bromine; In dichloromethane; at 20 - 45 ℃; for 0.916667h;
|
|
|
With pyridinium hydrobromide perbromide; In tetrahydrofuran; at 0 ℃; Reflux;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; In dichloromethane; at 0 ℃; for 0.166667h;
With bromine; In dichloromethane; for 2.75h; Reflux;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; In dichloromethane; at 20 ℃; for 0.25h;
With bromine; In dichloromethane; at 20 - 40 ℃; for 0.916667h;
|
|
|
5-acetyl-8-benzyloxy-1H-quinolin-2-one; With boron trifluoride diethyl etherate; In dichloromethane; Heating / reflux;
With bromine; In dichloromethane; for 4.5h; Heating / reflux;
With water; potassium carbonate; In dichloromethane; at 30 ℃; pH=8 - 9;
|
|
|
With boron trifluoride diethyl etherate; bromine; In dichloromethane; for 0.25h; Reflux;
|
-
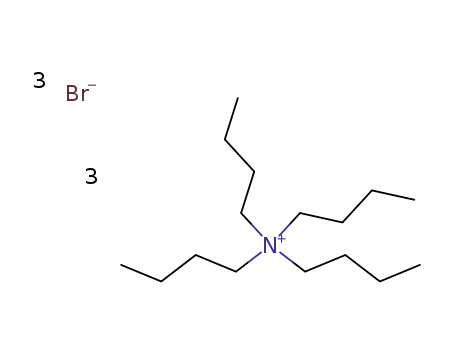
-
tetra-n-butylammonium tribromide

-

- 93609-84-8
5-acetyl-8-benzyloxy-1H-quinolin-2-one

-

- 100331-89-3
8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-one
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; methanol;
|
73% |
100331-89-3 Upstream products
-
93609-84-8

5-acetyl-8-benzyloxy-1H-quinolin-2-one
-
1127-45-3
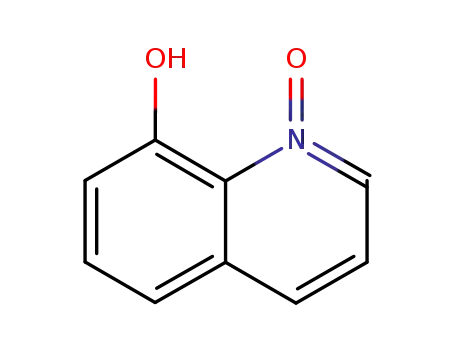
8-Hydroxyquinoline-N-oxide
-
148-24-3
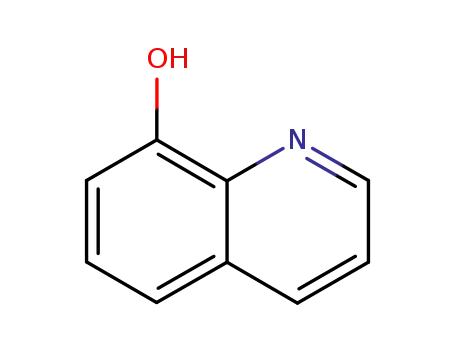
8-quinolinol
-
15450-72-3
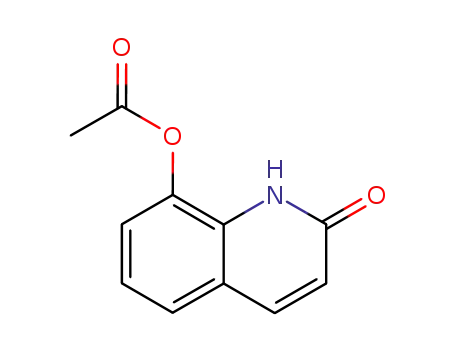
2-oxo-1,2-dihydroquinolin-8-yl acetate
100331-89-3 Downstream products
-
956234-40-5
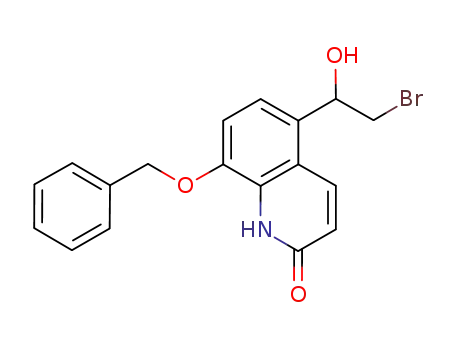
8-(benzyloxy)-5-(2-bromo-1-hydroxyethyl)quinolin-2(1H)-one
-
530084-79-8
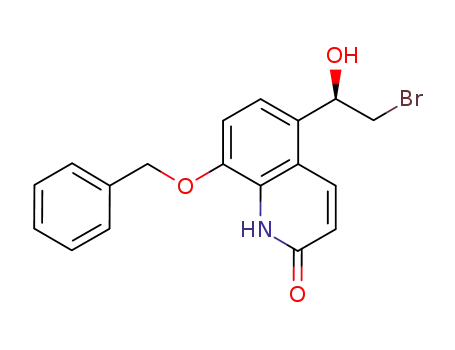
8-(benzyloxy)-5-[(1R)-2-bromo-1-hydroxyethyl]quinolin-2(1H)-one
-
743461-64-5
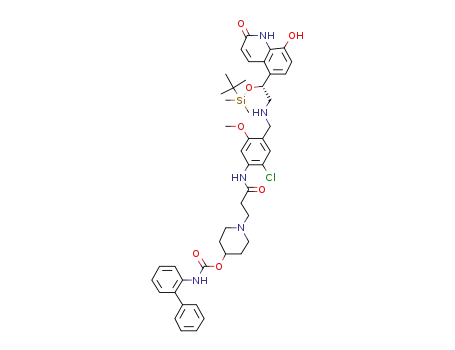
biphenyl-2-ylcarbamic Acid 1-[2-(4-{[(R)-2-(tert-Butyldimethylsilanyloxy)-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethylamino]methyl}-2-chloro-5-methoxy-phenylcarbamoyl)ethyl]piperidin-4-yl Ester
-
1042738-15-7
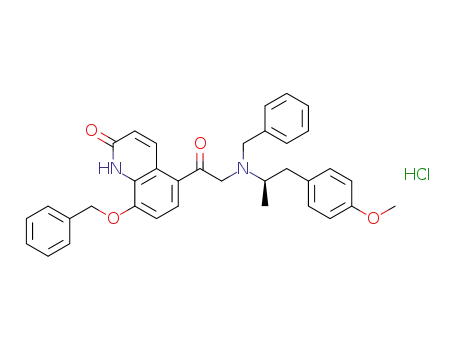
5-[[[(1R)-2-(4-methoxyphenyl)-1-methylethyl](phenylmethyl)amino]acetyl]-8-(phenylmethoxy)-(1H)-quinolin-2-one monohydrochloride
Relevant Products
-
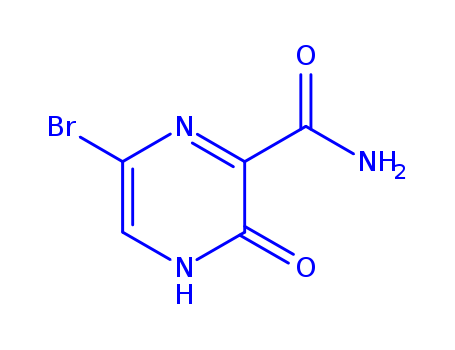
6-Bromo-3-hydroxypyrazine-2-carboxamide
CAS:259793-88-9
-
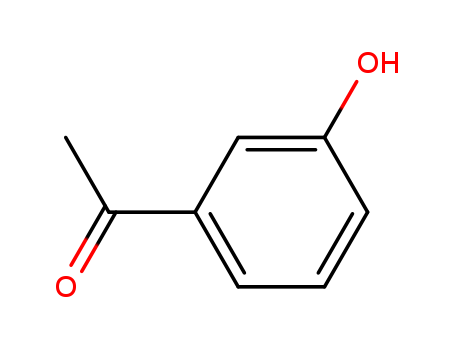
3-Hydroxyacetophenone
CAS:121-71-1
-
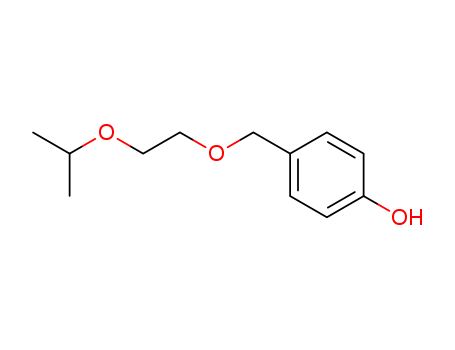
4-Isopropoxyethoxymethyl-1-Hydroxybenzene
CAS:177034-57-0