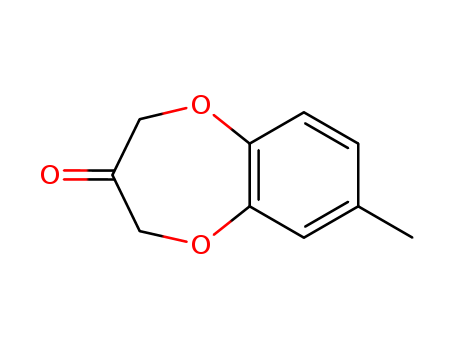
28940-11-6
- Product Name:7-methyl-2h-benzo[b][1,4]dioxepin-3(4h)-one
- Molecular Formula:C10H10O3
- Purity:99%
- Molecular Weight:178.188
Product Details;
CasNo: 28940-11-6
Molecular Formula: C10H10O3
Appearance: white crystalline powder
Buy Reliable Quality Sale 7-methyl-2h-benzo[b][1,4]dioxepin-3(4h)-one 28940-11-6 In Stock
- Molecular Formula:C10H10O3
- Molecular Weight:178.188
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0.000831mmHg at 25°C
- Melting Point:37.0 to 41.0 °C
- Refractive Index:1.538
- Boiling Point:305.2 °C at 760 mmHg
- Flash Point:129.3 °C
- PSA:35.53000
- Density:1.196 g/cm3
- LogP:1.33530
Watermelon Ketone(Cas 28940-11-6) Usage
|
Uses |
7-Methyl-2H-1,5-benzodioxepin-3(4H)-one has shown gender-specific reduction in overall olfactory impact of stress-related odor by cross adaptation in human apocrine secretion. |
|
Description |
Watermelon Ketone, also known as calone or methylbenodioxepinone is a hydrocarbon compound with similar structure of certain alicyclic C11-hydrocarbons such as ectocarpene. It is a flavor and fragrance chemical compound with special odorant. It was used as a scent component since the 1980s for its watery, fresh, ozone accords. It was later supplemented into several perfumes of the marine trend, beginning in the 1990s. |
|
Chemical Properties |
Watermelon Ketone is a white powder with a fresh marine odor, mp 35–41°C. It is used to create fresh aquatic marine notes in perfume oils for many applications, for example, for fine fragrances, soaps, and shower gels. |
|
Preparation |
The material is prepared by etherification of 4-methylpyrocatechol with two equivalents of alkyl 2-bromoacetate and subsequentDieckmann condensation followed by hydrolysis and decarboxylation. |
|
Flammability and Explosibility |
Nonflammable |
|
Trade name |
Aquamor (Aromor), Calone? (Firmenich), Ganone (Agan). |
InChI:InChI=1/C10H10O3/c1-7-2-3-9-10(4-7)13-6-8(11)5-12-9/h2-4H,5-6H2,1H3
28940-11-6 Relevant articles
KI-catalysed synthesis of 4-methylcatechol dimethylacetate and fragrant compound Calone 1951
Zhang, Ya-Zheng,Yang, Qian,Huang, Shao-Jian,Luo, Zi-Ping,Li, Wen-Ping,Dong, Li-Chun
, p. 586 - 593 (2013)
Synthesis of the fragrant compound Calon...
Microwave assisted synthesis of the fragrant compound Calone 1951
Drevermann, Britta,Lingham, Anthony,Hügel, Helmut,Marriott, Philip
, p. 39 - 41 (2005)
Microwave irradiation has been utilised ...
Method for synthesizing watermelon ketone
-
Paragraph 0034-0059, (2021/11/19)
The invention belongs to the technical f...
Preparation method of watermelon ketone
-
Paragraph 0059-0143, (2021/08/14)
The invention relates to a preparation m...
A Deoximation Method for Deprotection of Ketones and Aldhydes Using a Graphene-Oxide-Based Co-catalysts System
Tong, Qiaolin,Liu, Yang,Gao, Xuezhi,Fan, Zhanfang,Liu, Tianfu,Li, Bo,Su, Dangsheng,Wang, Qinghe,Cheng, Maosheng
supporting information, p. 3137 - 3145 (2019/05/01)
The deoximation of a wide range of ketox...
Watermelon ketone preparation method
-
, (2018/07/07)
The invention discloses a watermelon ket...
28940-11-6 Process route
-
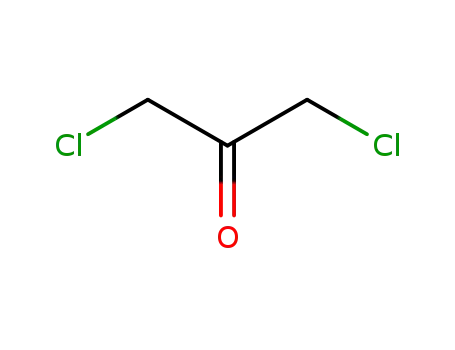
- 534-07-6
1,3-Dichloroacetone

-
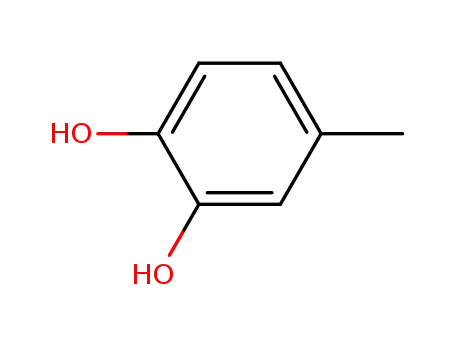
- 452-86-8
4-methyl-1,2-dihydroxybenzene

-
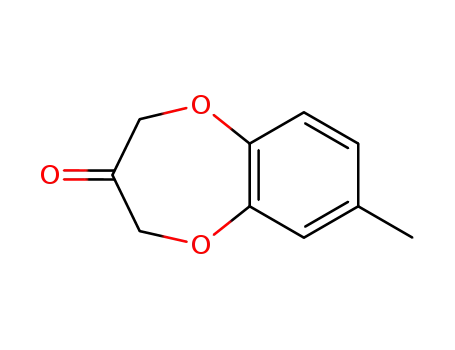
- 28940-11-6
7-methyl-3,4-dihydro-2H-1,5-benzodioxepin-3-one
| Conditions | Yield |
|---|---|
|
With sodium carbonate; potassium iodide; In butanone; for 4.16667h; Heating / reflux;
|
76.24% |
|
With sodium carbonate; potassium iodide; In cyclohexanone; for 4.41667h; Heating / reflux;
|
75.25% |
|
With sodium carbonate; potassium iodide; In acetone; at 45 - 55 ℃; for 12.5h; under 727.573 Torr; Reagent/catalyst; Solvent; Temperature; Pressure; Inert atmosphere;
|
68.9% |
|
With sodium carbonate; potassium iodide; In acetone; for 12h; Heating / reflux;
|
59.91% |
|
With potassium carbonate; sodium iodide; In acetone; for 4h; Heating;
|
51% |
|
With potassium carbonate; potassium iodide; In acetone;
|
10% |
|
With sodium hydrogencarbonate; potassium iodide; In acetone;
|
5% |
|
With caesium carbonate; potassium iodide; In acetone;
|
0% |
|
With sodium hydroxide; potassium iodide; In acetone;
|
0% |
|
With lithium carbonate; potassium iodide; In acetone;
|
0% |
-
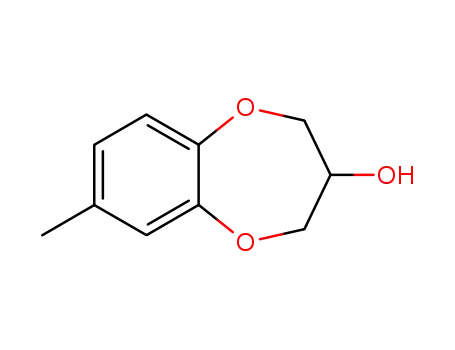
- 944558-65-0
(±)-7-methyl-3,4-dihydro-2H-1,5-benzodioxepin-3-ol

-

- 28940-11-6
7-methyl-3,4-dihydro-2H-1,5-benzodioxepin-3-one
| Conditions | Yield |
|---|---|
|
With 2-iodobenzenesulfonic acid; In nitromethane; at 25 ℃; for 6.5h; Solvent; Reagent/catalyst;
|
95% |
|
With potassium hydroxide; potassium permanganate; for 2.5h;
|
87% |
28940-11-6 Upstream products
-
52589-39-6
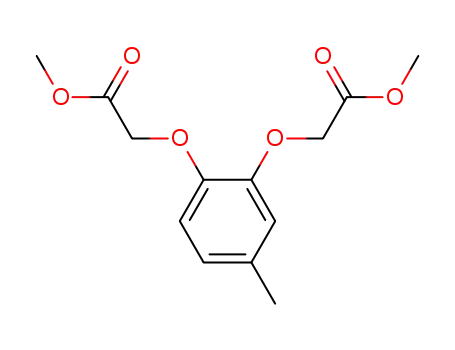
2,2'-[(4-methyl-1,2-phenylene)bis(oxy)]bis[acetic acid] dimethyl ester
-
944558-65-0

(±)-7-methyl-3,4-dihydro-2H-1,5-benzodioxepin-3-ol
-
534-07-6

1,3-Dichloroacetone
-
452-86-8

4-methyl-1,2-dihydroxybenzene
28940-11-6 Downstream products
-
1258873-82-3
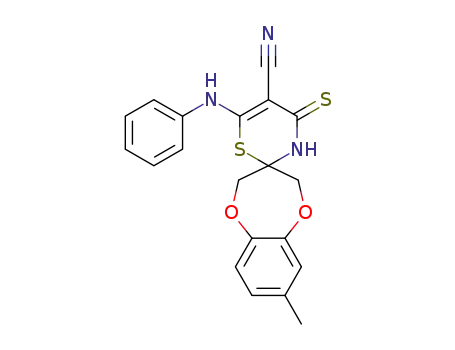
C20H17N3O2S2
-
1258873-68-5
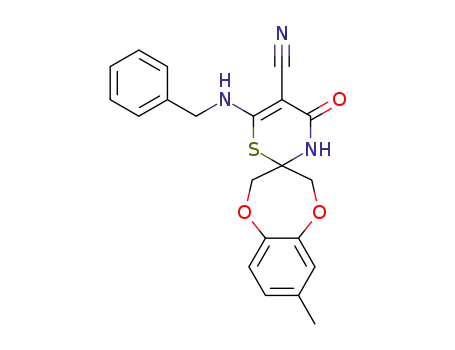
C21H19N3O3S
-
1258873-69-6
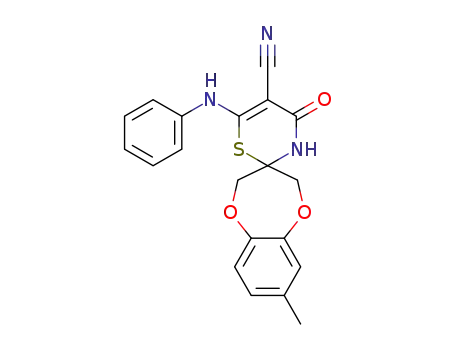
C20H17N3O3S
-
1258873-72-1
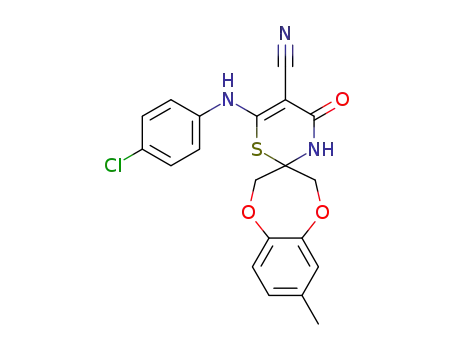
C20H16ClN3O3S
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
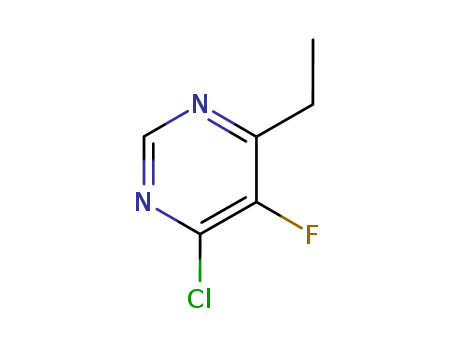
4-chloro-6-ethyl-5-fluoropyrimidine
CAS:137234-74-3
-
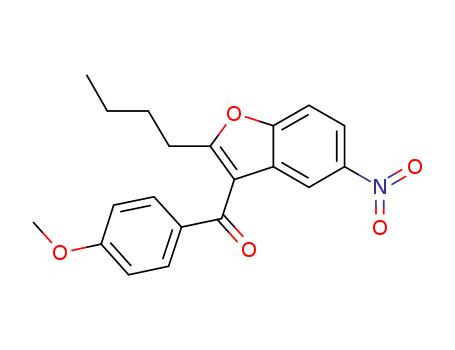
2-Butyl-3-(4-methoxybenzoyl)-5-nitrobenzofuran
CAS:141627-42-1