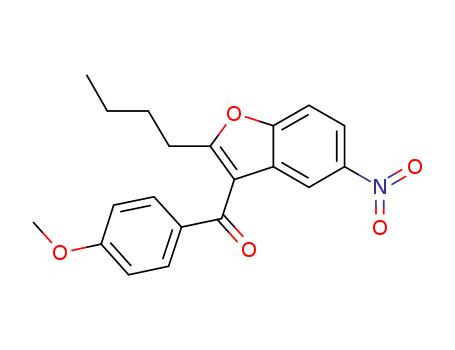
141627-42-1
- Product Name:2-Butyl-3-(4-methoxybenzoyl)-5-nitrobenzofuran
- Molecular Formula:C20H19NO5
- Purity:99%
- Molecular Weight:353.375
Product Details;
CasNo: 141627-42-1
Molecular Formula: C20H19NO5
Appearance: Off-white crystalline
Buy Quality Wholesale 2-Butyl-3-(4-methoxybenzoyl)-5-nitrobenzofuran 141627-42-1 Efficient Shipping
- Molecular Formula:C20H19NO5
- Molecular Weight:353.375
- Appearance/Colour:Off-white crystalline
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.603
- Boiling Point:537.726 °C at 760 mmHg
- Flash Point:279.007 °C
- PSA:85.26000
- Density:1.235 g/cm3
- LogP:5.44640
(2-Butyl-5-nitrobenzofuran-3-yl)(4-methoxyphenyl)methanone(Cas 141627-42-1) Usage
|
Uses |
(2-Butyl-5-nitrobenzofuran-3-yl)(4-methoxyphenyl)methanone, also known as (2-butyl-5-nitro-1-benzofuran-3-yl)-(4-methoxyphenyl)methanone, is a reagent in the preparation of heterocyclic benzofuran carboxamides via amidation of heterocyclic acyl chlorides with benzofuranamine. This is also a derivative of Dronedarone (D679445),which is a drug used for the treatment of atrial fibrillation and atrial flutter in patients who have suffered cardiac arrhythmias. |
InChIKey: WYALRXZJYXWYGR-UHFFFAOYSA-N
InChI: InChI=1S/C20H19NO5/c1-3-4-5-18-19(20(22)13-6-9-15(25-2)10-7-13)16-12-14(21(23)24)8-11-17(16)26-18/h6-12H,3-5H2,1-2H3
141627-42-1 Relevant articles
Discovery of dronedarone and its analogues as NLRP3 inflammasome inhibitors with potent anti-inflammation activity
Chen, Hao,Chen, Xiuhui,Sun, Ping,Wu, Dan,Yue, Hu,Pan, Jintao,Li, Xinxuan,Zhang, Cheng,Wu, Xinyi,Hua, Lei,Hu, Wenhui,Yang, Zhongjin
supporting information, (2021/06/18)
Inhibiting NLRP3 inflammasome activation...
Benzofuran compound and application thereof
-
Paragraph 0053-0055; 0062-0063, (2021/08/06)
The invention provides a benzofuran comp...
Electrochemical Cross-Dehydrogenative Coupling between Phenols and β-Dicarbonyl Compounds: Facile Construction of Benzofurans
Ding, Mengning,Shi, Zhuangzhi,Tian, Bailin,Wang, Yandong
, (2020/03/23)
Preparative electrochemical synthesis is...
Preparation method of key intermediate of dronedarone
-
Paragraph 0023; 0024, (2019/10/02)
The invention relates to a preparation m...
141627-42-1 Process route
-
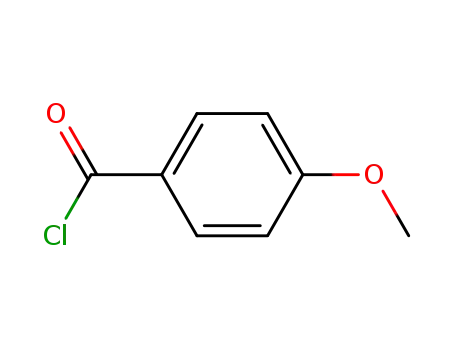
- 100-07-2
4-methoxy-benzoyl chloride

-
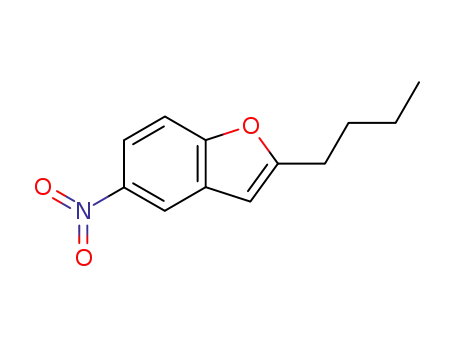
- 133238-87-6
2-(n-butyl)-5-nitrobenzofuran

-
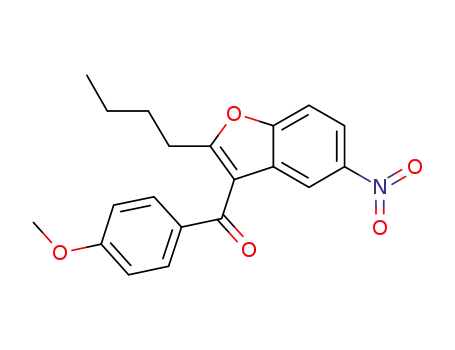
- 141627-42-1
(2-butyl-5-nitrobenzofuran-3-yl)(4-methoxyphenyl)methanone
| Conditions | Yield |
|---|---|
|
4-methoxy-benzoyl chloride; 2-(n-butyl)-5-nitrobenzofuran; aluminum (III) chloride; In chlorobenzene; at 25 ℃; for 2h; Reflux;
With water; In chlorobenzene; Product distribution / selectivity;
|
90% |
|
With aluminum (III) chloride; In dichloromethane; at 25 ℃; for 4h;
|
88% |
|
With tin(IV) chloride; In ice-water; 1,1-dichloroethane;
|
83.5% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 30 ℃; for 5.08333h; Concentration; Temperature;
|
82% |
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃; for 24h; Inert atmosphere;
|
78.9% |
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃; for 24h;
|
78.9% |
|
With aluminum (III) chloride; In dichloromethane;
|
|
|
With aluminum (III) chloride; In dichloromethane; at 25 - 30 ℃; for 22h; Large scale;
|
|
|
With aluminum (III) chloride; In 1,2-dichloro-ethane; at 35 - 40 ℃; Large scale;
|
-
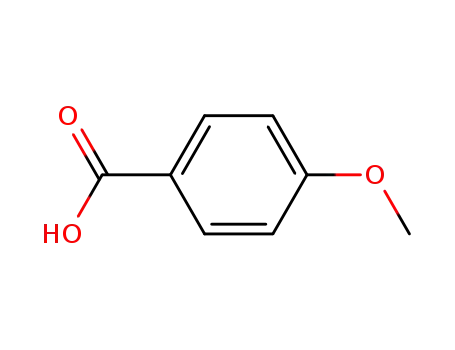
- 100-09-4
4-methoxybenzoic acid

-

- 133238-87-6
2-(n-butyl)-5-nitrobenzofuran

-

- 141627-42-1
(2-butyl-5-nitrobenzofuran-3-yl)(4-methoxyphenyl)methanone
| Conditions | Yield |
|---|---|
|
4-methoxybenzoic acid; 2-(n-butyl)-5-nitrobenzofuran; With Methyltrichlorosilane; iron(III) chloride; In chlorobenzene; at 20 - 40 ℃; for 4h;
With ethanol; In chlorobenzene; at 0 ℃;
|
72.7% |
141627-42-1 Upstream products
-
856758-03-7
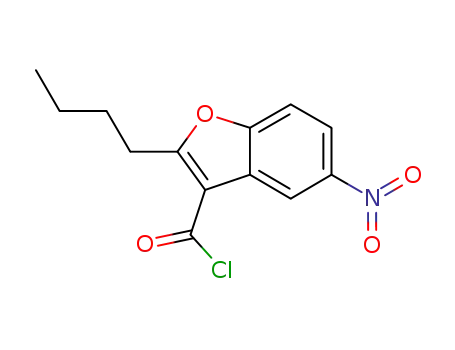
2-(n-butyl)-3-chlorocarbonyl-5-nitrobenzofuran
-
100-66-3
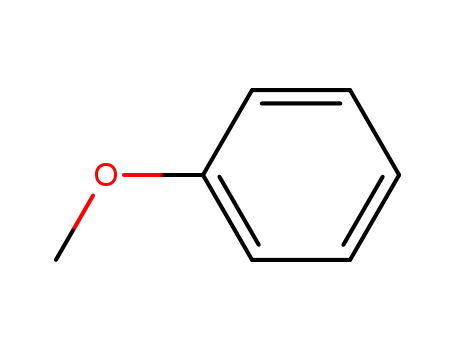
methoxybenzene
-
100-07-2

4-methoxy-benzoyl chloride
-
133238-87-6

2-(n-butyl)-5-nitrobenzofuran
141627-42-1 Downstream products
-
141645-16-1
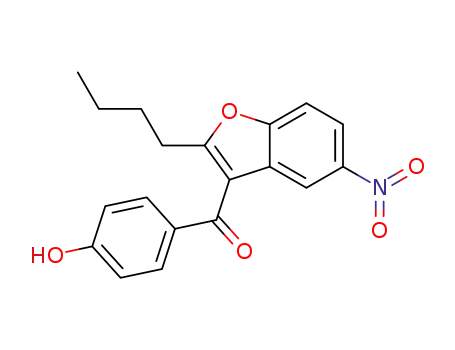
2-n-butyl-3-(4-hydroxybenzoyl)-5-nitrobenzofuran
-
856758-05-9
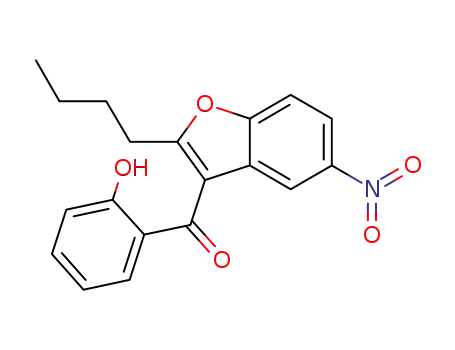
2-(n-butyl)-3-(2-hydroxybenzoyl)-5-nitrobenzofuran
-
1310430-05-7
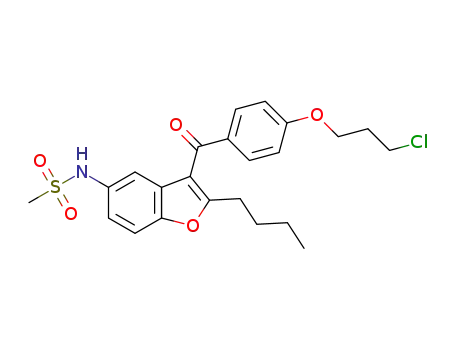
N-(2-butyl-3-(4-(3-chloropropoxy)benzoyl)benzofuran-5-yl)methanesulfonamide
-
1310430-03-5
(4-(3-chloropropoxy)phenyl)(2-butyl-5-nitrobenzofuran-3-yl)methanone
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
7-methyl-2h-benzo[b][1,4]dioxepin-3(4h)-one
CAS:28940-11-6
-
Medroxyprogesterone Acetate
CAS:71-58-9