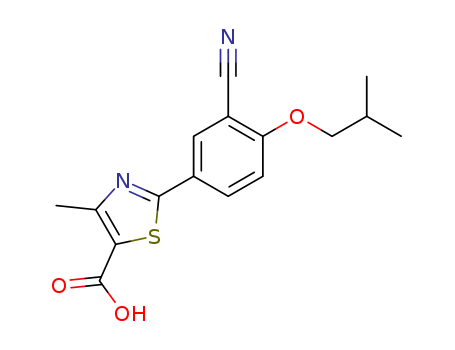
144060-53-7
- Product Name:Febuxostat
- Molecular Formula:C16H16N2O3S
- Purity:99%
- Molecular Weight:316.381
Product Details;
CasNo: 144060-53-7
Molecular Formula: C16H16N2O3S
Appearance: crystalline solid
Buy Quality Top Purity Febuxostat 144060-53-7 Fast Shipping
- Molecular Formula:C16H16N2O3S
- Molecular Weight:316.381
- Appearance/Colour:crystalline solid
- Vapor Pressure:2.41E-12mmHg at 25°C
- Melting Point:238-239 °C
- Refractive Index:1.605
- Boiling Point:536.6 °C at 760 mmHg
- PKA:2.48±0.10(Predicted)
- Flash Point:278.3 °C
- PSA:111.45000
- Density:1.31 g/cm3
- LogP:3.72318
Febuxostat(Cas 144060-53-7) Usage
|
Indications and Usage |
Febuxostat is a new generation xanthine oxidase inhibitor developed by Tejin Co. (Japan,) used clinically for for long-term treatment of hyperuicemia (gout,) a new and highly effective non-purine selective inhibitor of xanthine oxidase. It is not recommended for gout patients without hyperuricemia. |
|
Mechanisms of Action |
The production of uric acid in the body is related to purine metabolism. In the last step of the production, hypoxanthine produces xanthine through the action of xanthine oxidoreductase (XOR), finally producing uric acid. Inhibiting the activity of this enzyme can effectively reduce the production of uric acid. Xanthine oxidase is the main enzyme promoting uric acid production. Through highly selective inhibition of oxidized and reduced xanthine oxidase, Febuxostat can reduce synthesis of uric acid, decreasing its concentration and effectively treating gout . Through liver metabolism, Xanthine oxidase does not rely on renal excretion, so patients with moderate to severe liver and kidney dysfunction do not need to reduce dosages. Febuxostat is a non-purine XOR inhibitor, so it is very safe. |
|
Description |
Febuxostat is an antihyperuricemic nonpurine inhibitor of both the oxidized and reduced forms of xanthine oxidase. It inhibits bovine milk xanthine oxidase as well as mouse and rat liver xanthine oxidase/xanthine dehydrogenase (IC50s = 1.4, 1.8, and 2.2 nM, respectively). It is 10-30 times more potent than the hypoxanthine analog allopurinol (; Kis = 0.7 nM and 0.7 μM, respectively). Febuxostat decreases the serum level of urate in a potassium oxonate rat model of hyperuricemia (ED50 = 1.5 mg/kg). It reduces hepatic macrovesicular steatosis in mice fed a high-fat diet containing trans fatty acids when administered at a dose of 1 mg/kg per day. Febuxostat (0.75 mg/kg) also increases CNS expression of glutamate oxaloacetate transaminase 2 (GOT2) and improves neurological symptoms in a mouse model of secondary progressive experimental autoimmune encephalomyelitis (EAE). Formulations containing febuxostat have been used in the treatment of symptomatic hyperuricemia in patients with gout. |
|
Chemical Properties |
Crystalline Solid |
|
Originator |
Teijin (Japan) |
|
Uses |
antihyperlipidemic |
|
Brand name |
Uloric, Adenuric |
|
General Description |
Febuxostat is a potent, non-purine compound, which inhibits the expression of cytokines/chemokines. It has also been reported to inhibit LPS-induced TNF-α, VCAM-1, MMP9 and MCP-1 expression. |
|
Biochem/physiol Actions |
Febuxostat is a potent non-purine xanithine oxidase inhibitor. Febuxostat is used in urate lowering therapies (ULTs) for the treatment of gout. |
|
Clinical Use |
Fabuxostat was discovered by Teijin Pharmaceuticals and licensed to TAP Pharmaceuticals (which is currently part of Takeda Pharmaceuticals) and was approved in the U.S. for the treatment of hyperuricemia in patients with gout. It is a once-daily non-purine based agent with potent inhibitory activity against xanthine oxidase. The safety profile of the drug also does not require dose adjustment for patients with mild to moderate renal or hepatic impairment. Febuxostat is the first new agent cleared for this indication in 40 years. |
|
Synthesis |
There are a number of routes available to prepare this agent as discussed in recent publications. The synthesis shown in Scheme 10 is a short and concise route and does not require the use of toxic reagents. Thus the commercially available and easily prepared 4-hydroxythiobenzamide (52) was reacted with ethyl bromoacetoacetate (53) in refluxing ethanol to provide the thiazole ester 54 in ≈60% yield after crystallization. The phenolic ester 54 was then treated with hexamethylenetetramine (HMTA) in polyphosphoric acid at 80 °C to provide the crude aldehyde 55 (74% conversion by HPLC). Reaction of phenol 55 and isobutyl bromide (56) in the presence of potassium carbonate with catalytic potassium iodide in DMF gave isobutyl ether 57 (64%, two steps). This ether was then converted in one pot to nitrile 58 in 93% by reacting the aldehyde with hydroxylamine hydrochloride and sodium formate in refluxing formic acid. Saponification of the ester 58 with aqueous sodium hydroxide provided fabuxostat (X). |
|
Drug interactions |
Potentially hazardous interactions with other drugs Azathioprine: avoid concomitant use, increased risk of neutropenia. Cytotoxics: avoid concomitant use with mercaptopurine. Theophylline: use with caution |
|
Metabolism |
Extensively metabolised by conjugation via the uridine diphosphate glucuronosyltransferase (UDPGT) enzyme system, and by oxidation via the cytochrome P450 isoenzyme system to form active metabolites. About 49% of a dose is excreted via the urine, and 45% via the faeces (12% as unchanged drug) |
InChI:InChI=1/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20)
144060-53-7 Relevant articles
A facile one-pot synthesis of 4-alkoxy-1,3-benzenedicarbonitrile
Hasegawa, Masaichi
, p. 857 - 864 (1998)
2-(3-Cyano-4-isobutoxyphenyl)-4-methylth...
Synthesis, molecular docking, DFT study of novel N-benzyl-2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxamide derivatives and their antibacterial activity
Sam Daniel Prabu,Lakshmanan, Sivalingam,Thirumurugan,Ramalakshmi,Arul Antony
, p. 619 - 626 (2020)
A series of febuxostat based new chemica...
Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase
Yasuhiro Takano a, Kumiko Hase-Aoki a, Hideki Horiuchi a, Lin Zhao b, Yoshinori Kasahara a, Shiro Kondo a, Michael A. Becker c
, Life Sciences Volume 76, Issue 16, 4 March 2005, Pages 1835-1847
Febuxostat displayed potent mixed-type inhibition of the activity of purified bovine milk XO, with Ki and Ki′ values of 0.6 and 3.1 nM respectively, indicating inhibition of both the oxidized and reduced forms of XO.
144060-53-7 Process route
-
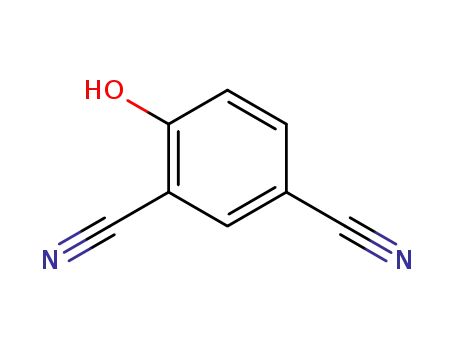
- 34133-58-9
4-hydroxy-isophthalonitrile

-
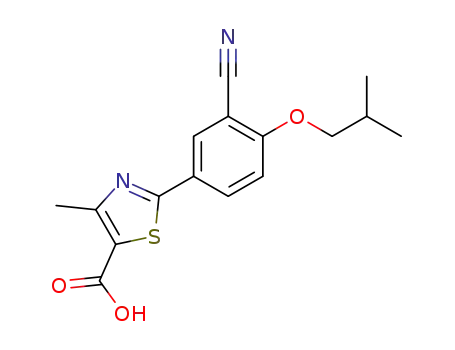
- 144060-53-7
febuxostat
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1: potassium carbonate; potassium iodide / dimethyl sulfoxide / 16 h / 70 - 75 °C
2: hydrogenchloride; thioacetamide / isopropyl alcohol / 14 h / 40 - 45 °C
3: ethyl acetate; N,N-dimethyl-formamide / 22 h / 80 - 85 °C
4: sodium hydroxide; ethanol / tetrahydrofuran / 1 h / 60 °C
With hydrogenchloride; ethanol; potassium carbonate; thioacetamide; potassium iodide; sodium hydroxide; In tetrahydrofuran; dimethyl sulfoxide; ethyl acetate; N,N-dimethyl-formamide; isopropyl alcohol;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate; potassium iodide / dimethyl sulfoxide / 16 h / 70 - 75 °C
2.1: hydrogenchloride; thioacetamide / isopropyl alcohol / 14 h / 40 - 45 °C
3.1: ethanol / 2 h / 100 °C
3.2: 1 h / 60 °C
With hydrogenchloride; potassium carbonate; thioacetamide; potassium iodide; In ethanol; dimethyl sulfoxide; isopropyl alcohol;
|
-
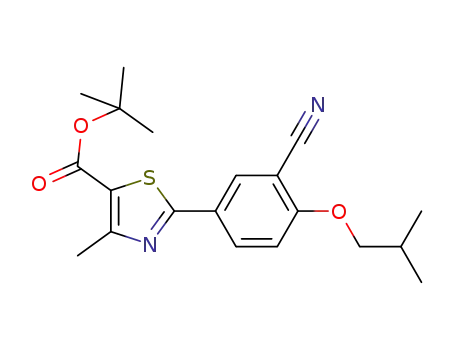
- 1139901-88-4
tert-butyl 2-(3’-cyano-4’-isobutoxyphenyl)-4-methylthiazole-5-carboxylate

-

- 144060-53-7
febuxostat
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 5h;
|
100% |
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 5h; Inert atmosphere;
|
100% |
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 18h;
|
93% |
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 18h;
|
93% |
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 2h;
|
90% |
144060-53-7 Upstream products
-
160844-75-7
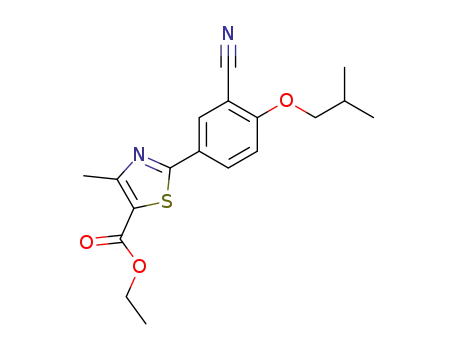
2-(3-cyano-4-isobutyloxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester
-
161718-81-6
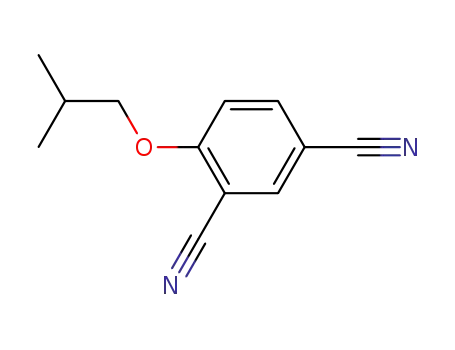
4-isobutoxyisophthalonitrile
-
163597-57-7
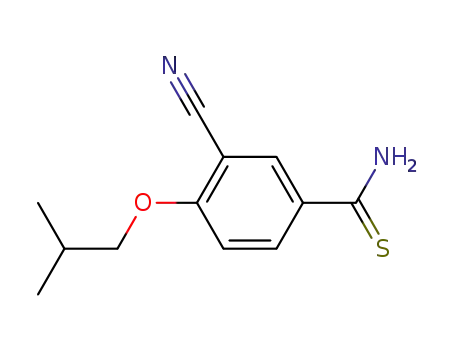
3-cyanoisobutoxybenzothioamide
-
619-72-7
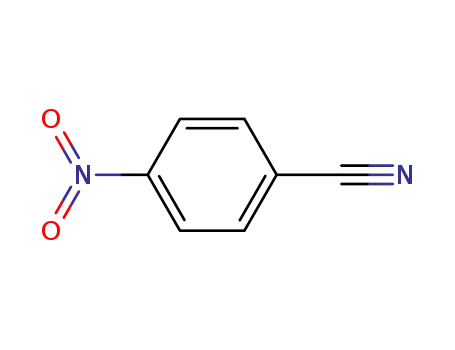
4-nitrobenzonitrile
144060-53-7 Downstream products
-
923942-34-1
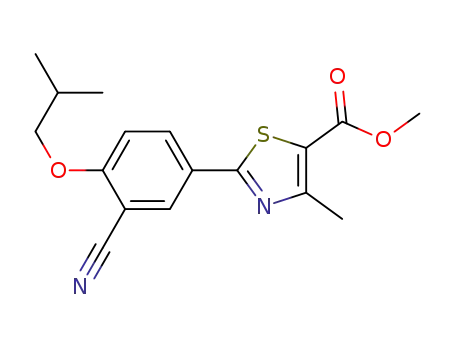
methyl 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate
-
923942-36-3
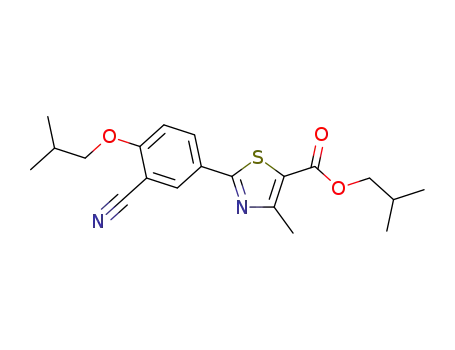
iso-butyl ester of febuxostat
Relevant Products
-
ethyl 2-(4-hydroxyphenyl)-4-methyl-5-Thiazolecarboxylate
CAS:161797-99-5
-
Zolmitriptan
CAS:139264-17-8
-
Ubrogepant Acid Intermediate
CAS:1375541-21-1