139264-17-8
- Product Name:Zolmitriptan
- Molecular Formula:C16H21N3O2
- Purity:99%
- Molecular Weight:287.362
Product Details;
CasNo: 139264-17-8
Molecular Formula: C16H21N3O2
Appearance: White or kind of white powder
Buy Reliable Quality Zolmitriptan 139264-17-8 Factory Sells In Stock
- Molecular Formula:C16H21N3O2
- Molecular Weight:287.362
- Appearance/Colour:White or kind of white powder
- Vapor Pressure:1.03E-12mmHg at 25°C
- Melting Point:136-141 °C
- Refractive Index:1.619
- Boiling Point:563.3 °C at 760 mmHg
- PKA:9.64(at 25℃)
- Flash Point:294.5 °C
- PSA:57.36000
- Density:1.217 g/cm3
- LogP:2.25170
Zolmitriptan(Cas 139264-17-8) Usage
|
Description |
Zolmitriptan is a selective serotonin receptor agonist of the 1B and 1D subtypes. It is mainly used in the acute treatment of migraine attacks with or without aura and cluster headaches. Zolmitriptan takes effect through binding to human 5-HT1Band 5-HT1Dreceptors, leading to cranial blood vessel constriction and the release of sensory neuropeptides through nerve endings in the trigeminal system. |
|
Chemical Properties |
White Crystalline Powder |
|
Originator |
Zeneca (UK) |
|
Uses |
Zolmitriptan is a serotonin 5HTID-receptor agonist and used to treat migraine (1,2,3). |
|
Brand name |
Zomig (AstraZeneca); Zomig (IPR). |
|
Therapeutic Function |
Serotoninergic |
|
General Description |
Zolmitriptan, the second triptan marketed (approved in1997), has a much better bioavailability (40%–48%) thansumatriptan. It is rapidly absorbed after oral or nasal sprayadministration. It also has an orally disintegrating tablet formulation(Zomig ZMT), which can be taken without water.Zolmitriptan undergoes rapid N-demethylation via CYP1A2to a more potent, active metabolite, N-desmethylzolmitriptan,which is 2 to 6 times more potent than the parentdrug. This active metabolite was detected 5 minutesafter dosing and accounts for about two thirds of the plasmaconcentration of the administered dose of the parent drug.284Thus, it is reasonable to assume that the therapeutic effectsand especially the CNS side effects of zolmitriptan must bein part attributed to the plasma levels of this active metabolite,at least until it is further degraded by hepatic MAO-Ato its inactive indole acetic acid derivatives. |
|
Biochem/physiol Actions |
Zolmitriptan is a selective serotonin receptor agonist of the 5HT1B and 5HT1D subtypes, both centrally and peripherally. It has been used clinically for the acute treatment of migraine attacks with or without aura and cluster headaches. |
|
Clinical Use |
Zomig was launched in Germany, Denmark, Sweden and the UK for use as an antimigraine agent (with and without aura). It can be prepared by three related routes of 5 to 7 steps starting from L-4-nitrophenylalanine. Zomig is a 5-HT1D/1B receptor agonist (10 fold ratio) with modest (< 100x) affinity for 5-HT1A and 5-HT1F receptors. It has no affinity for other serotonin receptors or receptors of other neurotransmitters. It has a novel dual action mechanism: centrally it acts on the trigeminal nucleus caudalis and peripherally is acts on the trigeminovascular system. Zomig was effective in treating headaches and nonheadache (photophobia, phonophobia and nausea) symptoms. It was 2-3 times more potent than sumatriptan and is metabolized to three compounds, one of which is 2-8 times more active than the parent. It caused a 40-50% decrease in headache after 1 h and a 73-77% after 4 h. There was a 30% reoccurance of headache but 90% effective treatment with a second dose. It blocks neurogenic inflammation by inhibiting release of peptides, causes vasoconstriction, and inhibits neuronal depolarization at peripheral sites in the cranium. It is 40% bioavailable and a 10 time theraputic dose showed no safety concerns. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: quinolones possibly inhibit metabolism - reduce dose of zolmitriptan. Antidepressants: increased risk of CNS toxicity with citalopram - avoid; risk of CNS toxicity with MAOIs and moclobemide - reduce dose of zolmitriptan to max 7.5 mg; SSRIs inhibit metabolism of zolmitriptan, reduce dose with fluvoxamine; possibly increased serotonergic effects with duloxetine and venlafaxine; increased serotonergic effects with St John’s wort - avoid concomitant use. Cimetidine: inhibits metabolism of zolmitriptan; maximum dose is 5 mg. Dapoxetine: possible increased risk of serotonergic effects - avoid for 2 weeks after stopping 5HT1 agonists. Ergot alkaloids: increased risk of vasospasm. Linezolid: risk of CNS toxicity - reduce dose of zolmitriptan. |
|
Metabolism |
Zolmitriptan is eliminated largely by hepatic biotransformation followed by urinary excretion of the metabolites. There are three major metabolites: the indole acetic acid, (the major metabolite in plasma and urine), the N-oxide and N-desmethyl analogues. Only the N-desmethylated metabolite is active. The primary metabolism of zolmitriptan is mediated mainly by the cytochrome P450 isoenzyme CYP1A2 while monoamine oxidase type A is responsible for further metabolism of the N-desmethyl metabolite. Over 60% of a dose is excreted in the urine, mainly as the indole acetic acid, and about 30% appears in the faeces, mainly as unchanged drug. |
InChI:InChI=1/C16H21N3O2/c1-19(2)6-5-12-9-17-15-4-3-11(8-14(12)15)7-13-10-21-16(20)18-13/h3-4,8-9,13,17H,5-7,10H2,1-2H3,(H,18,20)/t13-/m1/s1
139264-17-8 Relevant articles
Convenient and industrially viable process for preparation of zolmitriptan
Neelakandan,Manikandan,Santosha,Prabhakaran
, p. E332-E334 (2014)
The present invention provides a conveni...
Synthesis of zolmitriptan and related substances
Vujjini, Satish Kumar,Mothukuri, Vivekananda Reddy,Islam, Aminul,Bandichhor, Rakeshwar,Kagga, Mukkanti,Malakondaiah, Golla China
, p. 3294 - 3306 (2013)
Efficient and cost-effective synthesis o...
PROCESS FOR PREPARATION OF ZOLMITRIPTAN
-
Paragraph 0031-0032, (2014/08/19)
The present invention provides a conveni...
PROCESS FOR THE PREPARATION OF ZOLMITRIPTAN, SALTS AND SOLVATES THEREOF
-
Page/Page column 11, (2011/05/16)
The present invention relates to an impr...
139264-17-8 Process route
-
![(S)-3-(2-dimethylaminoethyl)-5-[2-oxo-1,3-oxazolidin-4-ylmethyl]-1H-indol-2-carboxylic acid](/upload/2023/9/26d8cf6a-e14f-40ec-8810-3fa95d8f2a76.png)
- 659738-69-9
(S)-3-(2-dimethylaminoethyl)-5-[2-oxo-1,3-oxazolidin-4-ylmethyl]-1H-indol-2-carboxylic acid

-
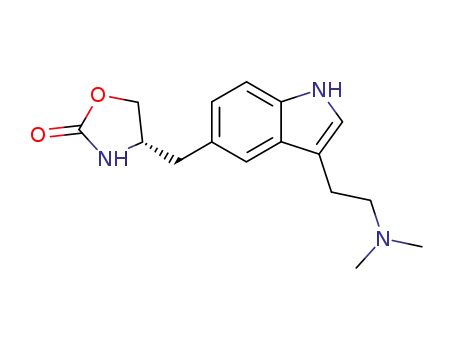
- 139264-17-8,139264-24-7,139264-82-7
zolmitriptan
| Conditions | Yield |
|---|---|
|
copper(II) oxide; In quinoline; at 200 ℃; for 0.333333h;
|
90% |
|
With hydrogenchloride; In water; at 15 - 18 ℃; Reflux; Industrial scale;
|
1.75 kg |
-
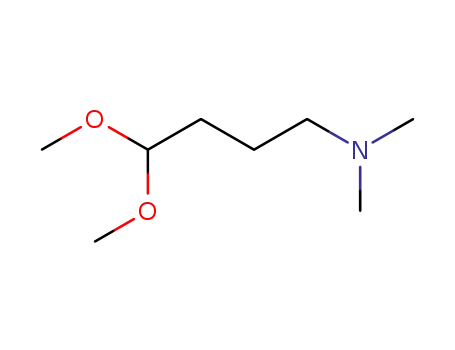
- 19718-92-4
4-(N,N-dimethylamino)butyraldehyde dimethyl acetal

-
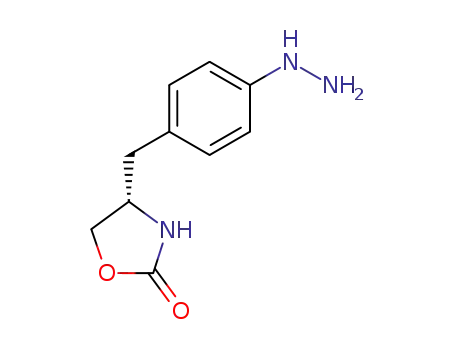
- 187975-62-8,171550-12-2
(S)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one

-

- 139264-17-8,139264-24-7,139264-82-7
zolmitriptan
| Conditions | Yield |
|---|---|
|
4-(N,N-dimethylamino)butyraldehyde dimethyl acetal; (S)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one; With hydrogenchloride; In water; at 25 - 90 ℃; for 5 - 6h; pH=2 - 9;
With sodium carbonate; In water; pH=~ 8 - 9;
|
|
|
4-(N,N-dimethylamino)butyraldehyde dimethyl acetal; (S)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one; In water; at 25 - 30 ℃; pH=9;
With hydrogenchloride; In water; at 85 - 90 ℃; pH=2;
With sodium carbonate; In water; pH=8 - 9;
|
139264-17-8 Upstream products
-
50-00-0
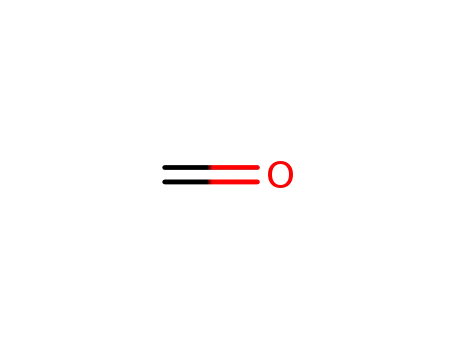
formaldehyd
-
139264-15-6
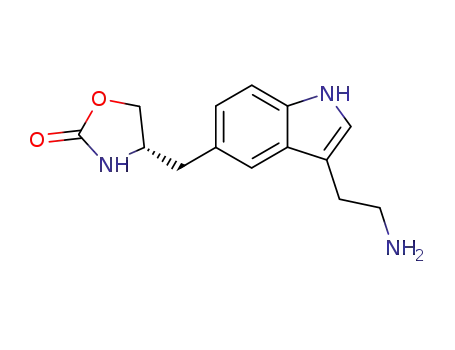
(S)-2-<5-<(2-oxo-1,3-oxazolidin-4-yl)methyl>-1H-indol-3-yl>ethylamine
-
1116-77-4
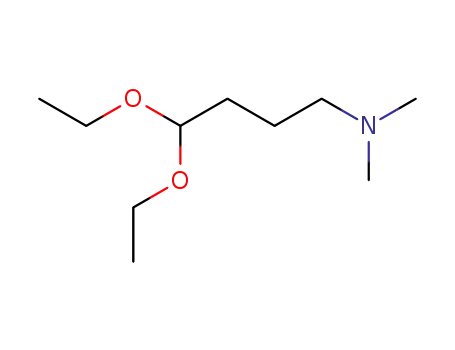
4,4-diethoxy-N,N-dimethyl-1-butanamine
-
139264-57-6
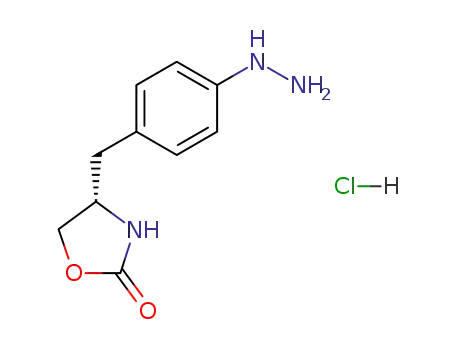
(S)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one hydrochloride
139264-17-8 Downstream products
-
251451-30-6
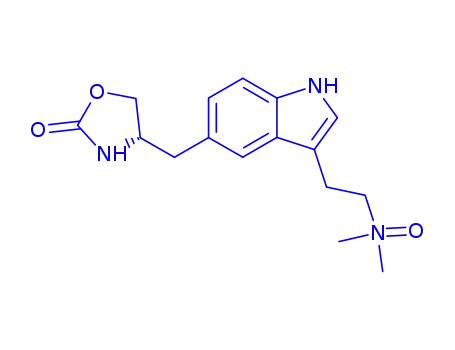
Zolmitriptan N-oxide
Relevant Products
-
Methyl trans-4-aminocyclohexanecarboxylate hydrochloride
CAS:61367-07-5
-
Algestone Acetophenide
CAS:24356-94-3
-
Febuxostat
CAS:144060-53-7