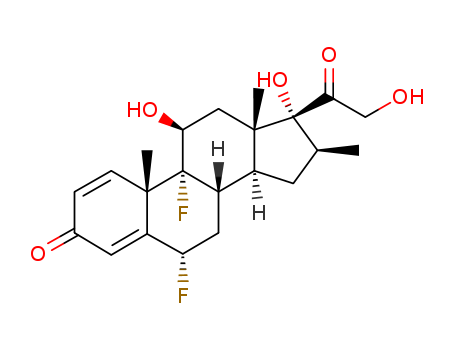
2557-49-5
- Product Name:Diflorasone
- Molecular Formula:C22H28F2O5
- Purity:99%
- Molecular Weight:410.458
Product Details;
CasNo: 2557-49-5
Molecular Formula: C22H28F2O5
Appearance: Off-white solid
Quality Factory Supply High Purity Diflorasone 2557-49-5 Reasonable Price
- Molecular Formula:C22H28F2O5
- Molecular Weight:410.458
- Appearance/Colour:Off-white solid
- Vapor Pressure:2.33E-15mmHg at 25°C
- Melting Point:228-239 °C
- Refractive Index:1.579
- Boiling Point:569.8 °C at 760 mmHg
- PKA:11.98±0.70(Predicted)
- Flash Point:298.4 °C
- PSA:94.83000
- Density:1.36 g/cm3
- LogP:1.84370
Diflorasone(Cas 2557-49-5) Usage
|
Chemical Properties |
Off-White Solid |
|
Originator |
Florone,Upjohn,US,1978 |
|
Uses |
An anti-inflammatory and anti-itching corticosteroid usually present in topical creams. |
|
Definition |
ChEBI: The 16beta-analogue of flumethasone. It is used as the 17,21-diacetate as a topical anti-inflammatory and antipruritic in the treatment of various skin disorders. |
|
Manufacturing Process |
6α-Fluoro-9β-epoxy-17α,21-dihydroxy-16α-methyl-1,4-pregnadiene-3,20- dione-21-acetate:To a solution of 6.78 g of 6α-fluoro-9α-bromo-11β,17α,21- trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21-acetate in 175 ml of acetone was added 6.78 g of potassium acetate and the resulting suspension was heated under reflux for a period of 17 hours. The mixture was then concentrated to approximately 60 ml volume at reduced pressure on the steam bath, diluted with water and extracted with methylene chloride. The methylene chloride extracts were combined, washed with water, dried over anhydrous sodium sulfate and evaporated. The residue was redissolved in methylene chloride and chromatographed over 500 g of Florisil anhydrous magnesium silicate. The column was eluted with 1 liter portions of hexanes (Skellysolve B) containing increasing proportions of acetone. There was so eluted 6α-fluoro-9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4- pregnadiene-3,20-dione-21-acetate which was freed of solvent by evaporation of the eluates.6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20- dione-2-1-acetate: To approximately 1.3 g of hydrogen fluoride contained in a polyethylene bottle and maintained at -60C was added 2.3 ml of tetrahydrofuran and then a solution of 500 mg (0,0012 mol) of 6α-fluoro9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4-pregnadiene-3,20-dione-21- acetate in two ml of methylene chloride. The steroid solution was rinsed in with an additional 1 ml of methylene chloride. The light red colored solution was then kept at approximately -30°C for 1 hour and at -10°C for 2 hours. At the end of this period it was mixed cautiously with an excess of cold sodium bicarbonate solution and the organic material extracted with the aid of additional methylene chloride.The combined extracts were washed with water, dried over anhydrous sodium sulfate and concentrated to approximately 35 ml. The solution was chromatographed over 130 g of Florisil anhydrous magnesium silicate. The column was developed with 260 ml portions of hexanes (Skellysolve B) containing increasing proportions of acetone. There was thus eluted 6α,9αdifluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21- acetate which was freed of solvent by evaporation of the eluate fractions.6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20- dione: 3.25 g of 6α,9α-difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4- pregnadiene-3,20-dione-21-acetate was dissolved in 325 ml of methanol, previously purged of air-oxygen by passing nitrogen through it for 10 minutes and thereto was added a solution of 1.63 g of potassium bicarbonate in 30 ml of water, similarly purged of oxygen. The mixture was allowed to stand at room temperature for a period of 5 hours in a nitrogen atmosphere, thereupon neutralized with 2.14 ml of acetic acid in 40 ml of water. The mixture was concentrated to approximately one-third volume at reduced pressure on a 60°C water bath. Thereupon 250 ml of water was added and the mixture chilled. The crystalline product was collected on a filter, washed with water and dried to give 6α,9α-difluoro-11β,17α,21-trihydroxy-16αmethyl-1,4-pregnadiene-3,20-dione.The diflorasone is reacted with orthoacetic acid trimethyl ester in the presence of toluenesulfonic acid to give diflorasone diacetate. |
|
Brand name |
Florone (Pharmacia & Upjohn); Psorcon (Pharmacia & Upjohn); Psorcon (Sanofi Aventis). |
|
Therapeutic Function |
Antiinflammatory |
InChI:InChI=1/C22H28F2O5/c1-11-6-13-14-8-16(23)15-7-12(26)4-5-19(15,2)21(14,24)17(27)9-20(13,3)22(11,29)18(28)10-25/h4-5,7,11,13-14,16-17,25,27,29H,6,8-10H2,1-3H3/t11-,13-,14-,16-,17-,19-,20-,21-,22-/m0/s1
2557-49-5 Relevant articles
A 17, 21 - double-hydroxy steroid derivatives of synthetic method
-
Paragraph 0042; 0043; 0045; 0047; 0072, (2018/04/02)
The invention relates to a method of pre...
A PROCESS FOR PREPARING A CRYSTALLINE FORM OF HALOBETASOL PROPIONATE
-
Page/Page column 25, (2008/06/13)
The present invention provides a process...
Method for the preparation of 6alpha-fluoro corticosteroids
-
, (2008/06/13)
A method for producing a 6α-fluorinated ...
Method for reducing or preventing transplant rejection in the eye and intraocular implants for use therefor
-
, (2008/06/13)
Methods for reducing or preventing trans...
2557-49-5 Process route
-
-
C25H36F2O5Si

-
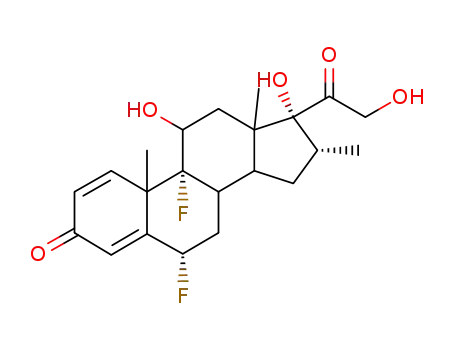
- 2135-17-3,2557-49-5,6477-56-1,59905-50-9,60895-22-9
6α-Fluor-dexamethason
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; water; pH=Ca. 1;
|
72.9 g |
-
-
C25H36F2O3Si

-

- 2135-17-3,2557-49-5,6477-56-1,59905-50-9,60895-22-9
6α-Fluor-dexamethason
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 3-chloro-benzenecarboperoxoic acid; potassium hydrogencarbonate / dichloromethane / 0 - 5 °C
2: hydrogenchloride / methanol; water / pH Ca. 1
With hydrogenchloride; potassium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid; In methanol; dichloromethane; water;
|
2557-49-5 Upstream products
-
84509-92-2
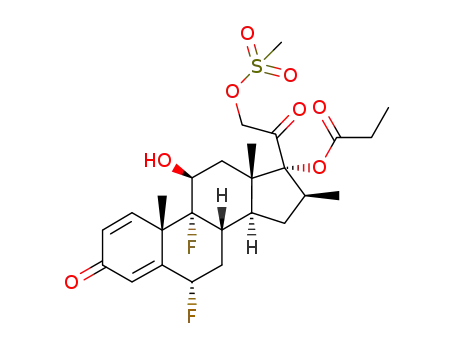
6α,9α-difluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione, 17-propionate 21-mesylate
2557-49-5 Downstream products
-
28416-82-2
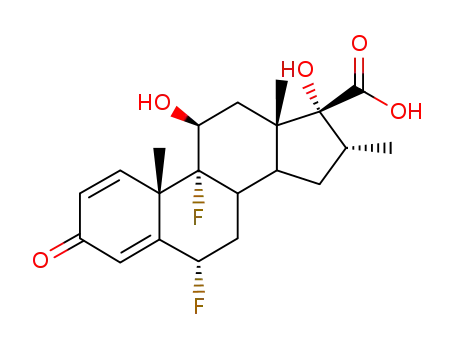
6α,9α-difluoro-11β,17α-dihydroxy-16α-methyl-3-oxo-androsta-1,4-diene-17β-carboxylic acid
-
28416-82-2
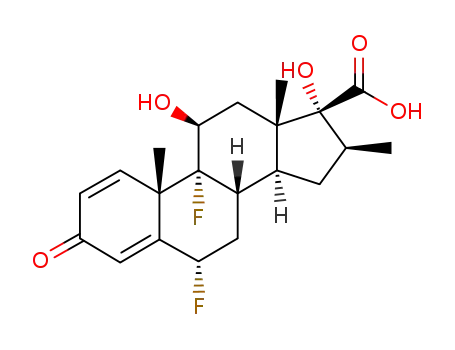
6α,9α-difluoro-11β,17α-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carboxylic acid
-
101916-29-4
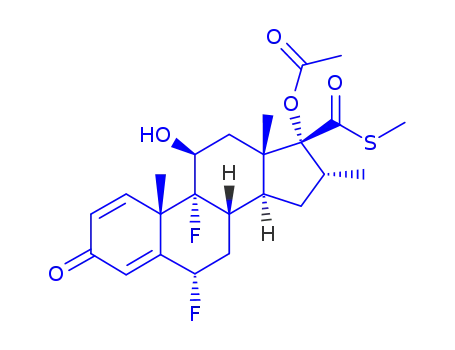
17α-acetoxy-6α,9α-difluoro-11β-hydroxy-17β-<(methylthio)carbonyl>-16α-methylandrosta-1,4-dien-3-one
-
65429-42-7
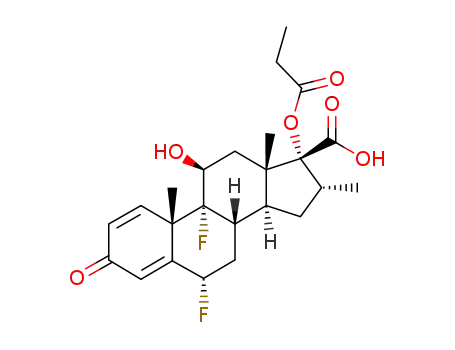
6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-(propionyloxy)androsta-1,4-diene-17β-carboxylic acid
Relevant Products
-
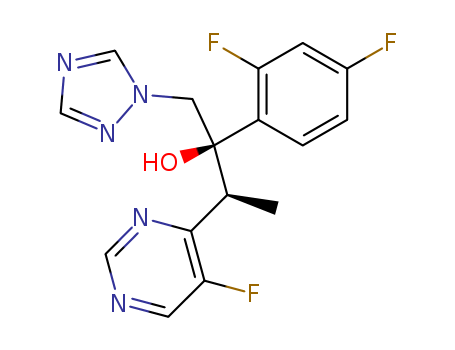
Voriconazole
CAS:137234-62-9
-
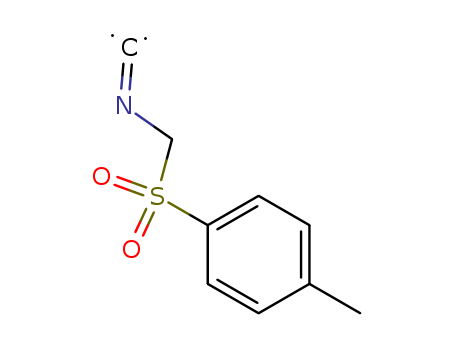
Tosylmethyl isocyanide
CAS:36635-61-7
-
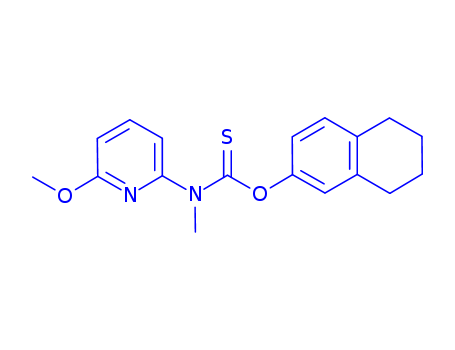
Liranaftate
CAS:88678-31-3