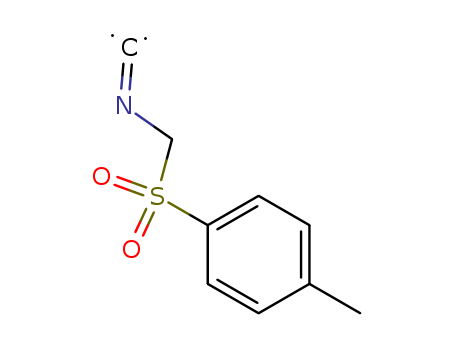
36635-61-7
- Product Name:Tosylmethyl isocyanide
- Molecular Formula:C9H9NO2S
- Purity:99%
- Molecular Weight:195.242
Product Details;
CasNo: 36635-61-7
Molecular Formula: C9H9NO2S
Appearance: Pale yellow to light brown crystalline powder
Buy Reliable Quality High Purity 99% Tosylmethyl isocyanide 36635-61-7 Efficient Transportation
- Molecular Formula:C9H9NO2S
- Molecular Weight:195.242
- Appearance/Colour:Pale yellow to light brown crystalline powder
- Melting Point:109-113 °C(lit.)
- Refractive Index:1.5270 (estimate)
- Boiling Point:400.9 °C at 760 mmHg
- Flash Point:196.3 °C
- PSA:42.52000
- Density:1.24 g/cm3
- LogP:1.95700
Tosylmethyl isocyanide(Cas 36635-61-7) Usage
|
Chemical Properties |
Pale yellow to light brown crystalline powder |
|
Uses |
Reagent for use in the preparation of biologically active pyrroles and imidazoles.1,2 |
|
Preparation |
N-(p-Tolylsulfonylmethyl)formamide 1609: A 3-L, three-necked, round-bottomed flask, equipped with a mechanical stirrer, a condenser, and a thermometer, was charged with sodium p-toluenesulfinate 1606 (267 g, 1.5 mol). After the addition of water (750 mL), a 34–37% solution of formaldehyde 1607 (350 mL, 378 g, ca. 4.4 mol), formamide 1608 (600 mL, 680 g, 15 mol), and formic acid (200 mL, 244 g, 5.3 mol), the stirred reaction mixture was heated at 90 C°. The sodium p-toluenesulfinate dissolved during the heating, and the clear solution was kept at 90–95 C° for 2 h. It was then cooled in an ice/salt bath with continued stirring and further cooled overnight in a freezer at 20 C°. The white solid produced was collected by suction filtration. It was washed thoroughly in a beaker by stirring with three 250 mL portions of iced water. The product was dried under reduced pressure over phosphorus pentoxide at 70 C° to provide 134–150 g (42–47%) of crude N-(p-tolylsulfonylmethyl)formamide 1609; mp 106–110 C°. This product was sufficiently pure to be used directly in the following reaction. |
|
Purification Methods |
Use an efficient fume cupboard. Purify TOSMIC by dissolving (50g) in CH2Cl2 (150mL) and passing it through a column (40x3cm) containing neutral alumina (100g) in CH2Cl2 and eluting with CH2Cl2. A nearly colourless solution (700mL) is collected, evaporated in vacuo and the residue (42-47g) of TOSMIC (m 113-114o dec) is recrystallised once from MeOH (m 116-117o dec). [Hoogenboom et al. Org Synth 57 102 1977, Lensen Tetrahedron Lett 2367 1972.] It also crystallises from EtOH (charcoal) [Saito & Itano, J Chem Soc, Perkin Trans 1 1 1986]. |
InChI:InChI=1/C9H9NO2S/c1-8-3-5-9(6-4-8)13(11,12)7-10-2/h3-6H,7H2,1H3
36635-61-7 Relevant articles
Mechanochemical Syntheses of N-Containing Heterocycles with TosMIC
Bolm, Carsten,Molitor, Claude,Rissanen, Kari,Schumacher, Christian,Smid, Sabrina,Truong, Khai-Nghi
, p. 14213 - 14222 (2021/09/07)
A mechanochemical van Leusen pyrrole syn...
Silver-Catalyzed Acyl Nitrene Transfer Reactions Involving Dioxazolones: Direct Assembly of N-Acylureas
Yang, Zheng-Lin,Xu, Xin-Liang,Chen, Xue-Rong,Mao, Zhi-Feng,Zhou, Yi-Feng
supporting information, p. 648 - 652 (2020/12/21)
Dioxazolones and isocyanides are useful ...
Phenyl pyrrole compound and application thereof in bactericidal activity
-
Paragraph 0137; 0140, (2020/08/17)
The invention provides a novel phenyl py...
Nitrogen-substituted phenyl pyrrole compound and application thereof in plant sterilization
-
Paragraph 0143; 0152; 155, (2020/07/21)
The invention provides a novel nitrogen-...
36635-61-7 Process route
-

- 36635-56-0
N-(4-methylbenzenesulfonylmethyl)-formamide

-
![[(p-methylphenyl)sulfonylmethyl]isonitrile](/upload/2023/9/88c554b5-8596-440b-8be6-94b209416960.png)
- 38622-91-2,36635-61-7
[(p-methylphenyl)sulfonylmethyl]isonitrile
| Conditions | Yield |
|---|---|
|
With triethylamine; trichlorophosphate; In tetrahydrofuran; diethyl ether; at 0 ℃; for 0.5h;
|
95% |
|
With triethylamine; trichlorophosphate; In dichloromethane; at -3 - 0 ℃; for 1h;
|
80% |
|
With triethylamine; trichlorophosphate; In dichloromethane; at -3 - 0 ℃; for 1h;
|
80% |
|
With diisopropylamine; trichlorophosphate; In dichloromethane;
|
68% |
|
N-(4-methylbenzenesulfonylmethyl)-formamide; With diisopropylamine; sodium chloride; trichlorophosphate; In tetrahydrofuran; at -20 ℃; for 1h; Inert atmosphere;
In tetrahydrofuran; at 0 ℃; for 1h;
|
64% |
|
With hexabromoethane; triethylamine; triphenylphosphine; In 1,2-dichloro-ethane; at -10 ℃; for 0.0166667h; Product distribution; other reagents, other temp., other time;
|
61% |
|
With hexabromoethane; triethylamine; triphenylphosphine; In 1,2-dichloro-ethane; at -10 ℃; for 0.0166667h;
|
61% |
|
With triethylamine; In dichloromethane;
|
|
|
With triethylamine; trichlorophosphate;
|
|
|
With triethylamine; trichlorophosphate; In dichloromethane;
|
|
|
With diisopropylamine; trichlorophosphate; In tetrahydrofuran; acetonitrile; at -20 - 5 ℃; for 1h; Inert atmosphere;
|
0.406 g |
|
N-(4-methylbenzenesulfonylmethyl)-formamide; With triethylamine; trichlorophosphate; In tetrahydrofuran; at -5 ℃; for 2h;
With water; In tetrahydrofuran; at 0 ℃; for 0.5h;
|
2 g |
|
With triethylamine; trichlorophosphate; In dichloromethane; at -4 ℃; for 3h; Temperature;
|
40.6 g |
|
With triethylamine; trichlorophosphate; In dichloromethane; at -5 - 0 ℃; Inert atmosphere;
|
|
|
With trichlorophosphate; In dichloromethane; at -5 - 0 ℃; for 5h; Temperature;
|
156 g |
|
With triethylamine; trichlorophosphate; In dichloromethane; at -30 ℃; for 3h;
|
-

- 60-29-7,927820-24-4
diethyl ether

-

- 129747-73-5
3-thiophenecarboxaldehyde

-

- 82072-22-8
3-hydroxymethylbenzyl bromide

-

-
3-[3-(5-oxazolyl)phenylthiomethyl)benzyl alcohol

-
![[(p-methylphenyl)sulfonylmethyl]isonitrile](/upload/2023/9/88c554b5-8596-440b-8be6-94b209416960.png)
- 38622-91-2,36635-61-7
[(p-methylphenyl)sulfonylmethyl]isonitrile
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In N-methyl-acetamide; water;
|
84% |
36635-61-7 Upstream products
-
593-75-9

methyl isocyanate
-
455-16-3

p-toluenesulfonyl fluoride
-
36635-56-0

N-(4-methylbenzenesulfonylmethyl)-formamide
-
60-29-7

diethyl ether
36635-61-7 Downstream products
-
70380-73-3

2-(1,3-oxazol-5-yl)pyridine
-
70380-75-5

4-(oxazol-5-yl)pyridine
-
70380-74-4

3-(oxazol-5-yl)pyridine
-
64269-61-0

5-butylsulfanyl-4-(toluene-4-sulfonyl)-thiazole
Relevant Products
-
Febuxostat
CAS:144060-53-7
-
Methylprednisolone
CAS:83-43-2
-
Diflorasone
CAS:2557-49-5